Nucleotide release sequences in the protein kinase SRPK1 accelerate substrate phosphorylation
- PMID: 22839969
- PMCID: PMC3718016
- DOI: 10.1021/bi300876h
Nucleotide release sequences in the protein kinase SRPK1 accelerate substrate phosphorylation
Abstract
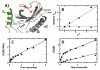
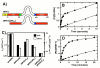
Protein kinases are essential signaling enzymes that transfer phosphates from bound ATP to select amino acids in protein targets. For most kinases, the phosphoryl transfer step is highly efficient, while the rate-limiting step for substrate processing involves slow release of the product ADP. It is generally thought that structural factors intrinsic to the kinase domain and the nucleotide-binding pocket control this step and consequently the efficiency of protein phosphorylation for these cases. However, the kinase domains of protein kinases are commonly flanked by sequences that regulate catalytic function. To address whether such sequences could alter nucleotide exchange and, thus, regulate protein phosphorylation, the presence of activating residues external to the kinase domain was probed in the serine protein kinase SRPK1. Deletion analyses indicate that a small segment of a large spacer insert domain and a portion of an N-terminal extension function cooperatively to increase nucleotide exchange. The data point to a new mode of protein kinase regulation in which select sequences outside the kinase domain constitute a nucleotide release factor that likely interacts with the small lobe of the kinase domain and enhances protein substrate phosphorylation through increases in ADP dissociation rate.
Figures





References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. - PubMed
-
- Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4:E127–130. - PubMed
-
- Soderling TR. Protein kinases. Regulation by autoinhibitory domains. J Biol Chem. 1990;265:1823–1826. - PubMed
-
- Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich NP. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex [see comments] Nature. 1995;376:313–320. - PubMed
-
- Hagopian JC, Kirtley MP, Stevenson LM, Gergis RM, Russo AA, Pavletich NP, Parsons SM, Lew J. Kinetic Basis for Activation of CDK2/Cyclin A by Phosphorylation. J Biol Chem. 2001;276:275–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

