PET imaging of tumor associated macrophages using mannose coated 64Cu liposomes
- PMID: 22840225
- PMCID: PMC6547362
- DOI: 10.1016/j.biomaterials.2012.07.022
PET imaging of tumor associated macrophages using mannose coated 64Cu liposomes
Abstract
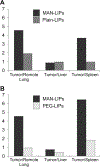
Macrophages within the tumor microenvironment (TAMs) have been shown to play a major role in the growth and spread of many types of cancer. Cancer cells produce cytokines that cause macrophages to express scavenger receptors (e.g. the mannose receptor) and factors that facilitate tissue and blood vessel growth, suppress T cell mediated anti-tumor activity, and express enzymes that can break down the extracellular matrix, thereby promoting metastasis. We have designed a mannosylated liposome (MAN-LIPs) and show that it accumulates in TAMs in a mouse model of pulmonary adenocarcinoma. These liposomes are loaded with (64)Cu to allow tracking by PET imaging, and contain a fluorescent dye in the lipid bilayer permitting subsequent fluorescence microscopy. We injected these liposomes into a mouse model of lung cancer. In vivo PET images were acquired 6 h after injection followed by the imaging of select excised organs. MAN-LIPs accumulated in TAMs and exhibited little accumulation in remote lung areas. MAN-LIPs are a promising new vehicle for the delivery of imaging agents to lung TAMs. In addition to imaging, MAN-LIPs hold the potential for delivery of therapeutic agents to the tumor microenvironment.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures






References
-
- Marin L, Colombo P, Bebawy M, Young PM, Traini D, Marin L, et al. Chronic obstructive pulmonary disease: patho-physiology, current methods of treatment and the potential for simvastatin in disease management. Expert Opin Drug Deliv 2011;8(9):1205–20. - PubMed
-
- Murphy BS, Bush HM, Sun dareshan V, Davis C, Hagadone J, Cory TJ, et al. Characterization of macrophage activation states in patients with cystic fibrosis. J Cyst Fibros 2010;9(5):314–22. - PubMed
-
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A, Allavena P, et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 2008;66(1):1–9. - PubMed
-
- Bingle L, Brown NJ, Lewis CE, Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anti-cancer therapies. J Pathol 2002;196(3):254–65. - PubMed
-
- Murdoch C, Muthana M, Coffelt SB, Lewis CE, Murdoch C, Muthana M, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8(8):618–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

