Differential trafficking of transport vesicles contributes to the localization of dendritic proteins
- PMID: 22840400
- PMCID: PMC3408588
- DOI: 10.1016/j.celrep.2012.05.018
Differential trafficking of transport vesicles contributes to the localization of dendritic proteins
Abstract
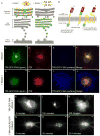
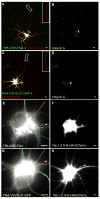
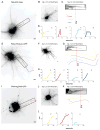
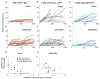
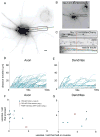
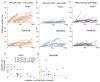
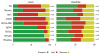
In neurons, transmembrane proteins are targeted to dendrites in vesicles that traffic solely within the somatodendritic compartment. How these vesicles are retained within the somatodendritic domain is unknown. Here, we use a novel pulse-chase system, which allows synchronous release of exogenous transmembrane proteins from the endoplasmic reticulum to follow movements of post-Golgi transport vesicles. Surprisingly, we found that post-Golgi vesicles carrying dendritic proteins were equally likely to enter axons and dendrites. However, once such vesicles entered the axon, they very rarely moved beyond the axon initial segment but instead either halted or reversed direction in an actin and Myosin Va-dependent manner. In contrast, vesicles carrying either an axonal or a nonspecifically localized protein only rarely halted or reversed and instead generally proceeded to the distal axon. Thus, our results are consistent with the axon initial segment behaving as a vesicle filter that mediates the differential trafficking of transport vesicles.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

