The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult
- PMID: 22840402
- PMCID: PMC3408587
- DOI: 10.1016/j.celrep.2012.06.003
The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult
Abstract
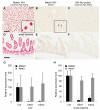
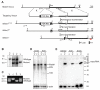
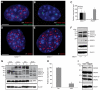
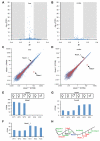
Genome-wide studies have identified thousands of long noncoding RNAs (lncRNAs) lacking protein-coding capacity. However, most lncRNAs are expressed at a very low level, and in most cases there is no genetic evidence to support their in vivo function. Malat1 (metastasis associated lung adenocarcinoma transcript 1) is among the most abundant and highly conserved lncRNAs, and it exhibits an uncommon 3'-end processing mechanism. In addition, its specific nuclear localization, developmental regulation, and dysregulation in cancer are suggestive of it having a critical biological function. We have characterized a Malat1 loss-of-function genetic model that indicates that Malat1 is not essential for mouse pre- and postnatal development. Furthermore, depletion of Malat1 does not affect global gene expression, splicing factor level and phosphorylation status, or alternative pre-mRNA splicing. However, among a small number of genes that were dysregulated in adult Malat1 knockout mice, many were Malat1 neighboring genes, thus indicating a potential cis-regulatory role of Malat1 gene transcription.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Camblong J, Iglesias N, Fickentscher C, Dieppois G, Stutz F. Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae. Cell. 2007;131:706–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

