Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression
- PMID: 22840761
- PMCID: PMC3725185
- DOI: 10.1016/j.biopsych.2012.06.022
Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression
Abstract
Background: Ketamine is reported to have rapid antidepressant effects; however, there is limited understanding of the time-course of ketamine effects beyond a single infusion. A previous report including 10 participants with treatment-resistant major depression (TRD) found that six ketamine infusions resulted in a sustained antidepressant effect. In the current report, we examined the pattern and durability of antidepressant effects of repeated ketamine infusions in a larger sample, inclusive of the original.
Methods: Participants with TRD (n = 24) underwent a washout of antidepressant medication followed by a series of up to six IV infusions of ketamine (.5 mg/kg) administered open-label three times weekly over a 12-day period. Participants meeting response criteria were monitored for relapse for up to 83 days from the last infusion.
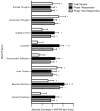
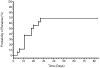
Results: The overall response rate at study end was 70.8%. There was a large mean decrease in Montgomery-Åsberg Depression Rating Scale score at 2 hours after the first ketamine infusion (18.9 ± 6.6, p < .001), and this decrease was largely sustained for the duration of the infusion period. Response at study end was strongly predicted by response at 4 hours (94% sensitive, 71% specific). Among responders, median time to relapse after the last ketamine infusion was 18 days.
Conclusions: Ketamine was associated with a rapid antidepressant effect in TRD that was predictive of a sustained effect. Future controlled studies will be required to identify strategies to maintain an antidepressant response among patients who benefit from a course of ketamine.
Trial registration: ClinicalTrials.gov NCT00548964.
Keywords: Antidepressant; experimental therapeutics; glutamate; ketamine; major depressive disorder; treatment-resistant depression.
Copyright © 2013 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Drs. Perez, Parides and aan het Rot and Ms. Pillemer, Stern and Collins reported no biomedical financial interests or potential conflicts of interest.
Figures

Comment in
-
Exploiting N-methyl-d-aspartate channel blockade for a rapid antidepressant response in major depressive disorder.Biol Psychiatry. 2013 Aug 15;74(4):238-9. doi: 10.1016/j.biopsych.2013.05.029. Biol Psychiatry. 2013. PMID: 23885752 No abstract available.
References
-
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. - PubMed
-
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining Medications to Enhance Depression Outcomes (CO-MED): Acute and Long-Term Outcomes of a Single-Blind Randomized Study. Am J Psychiatry. 2011;168:689–701. - PubMed
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. - PubMed
-
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

