Arginine topology controls escape of minimally cationic proteins from early endosomes to the cytoplasm
- PMID: 22840770
- PMCID: PMC3488872
- DOI: 10.1016/j.chembiol.2012.05.022
Arginine topology controls escape of minimally cationic proteins from early endosomes to the cytoplasm
Abstract
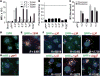
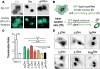
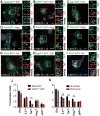
Proteins represent an expanding class of therapeutics, but their actions are limited primarily to extracellular targets because most peptidic molecules fail to enter cells. Here we identified two small proteins, miniature protein 5.3 and zinc finger module ZF5.3, that enter cells to reach the cytosol through rapid internalization and escape from Rab5+ endosomes. The trafficking pathway mapped for these molecules differs from that of Tat and Arg(8), which require transport beyond Rab5+ endosomes to gain cytosolic access. Our results suggest that the ability of 5.3 and ZF5.3 to escape from early endosomes is a unique feature and imply the existence of distinct signals, encodable within short sequences, that favor early versus late endosomal release. Identifying these signals and understanding their mechanistic basis will illustrate how cells control the movement of endocytic cargo and may allow researchers to engineer molecules to follow a desired delivery pathway for rapid cytosolic access.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

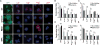

References
-
- Belitsky JM, Leslie SJ, Arora PS, Beerman TA, Dervan PB. Cellular uptake of N-methylpyrrole/N-methylimidazole polyamide-dye conjugates. Bioorg Med Chem. 2002;10:3313–3318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

