Staphylococcus aureus α-toxin modulates skin host response to viral infection
- PMID: 22840852
- PMCID: PMC3594992
- DOI: 10.1016/j.jaci.2012.06.019
Staphylococcus aureus α-toxin modulates skin host response to viral infection
Abstract
Background: Patients with atopic dermatitis (AD) with a history of eczema herpeticum have increased staphylococcal colonization and infections. However, whether Staphylococcus aureus alters the outcome of skin viral infection has not been determined.
Objective: We investigated whether S aureus toxins modulated host response to herpes simplex virus (HSV) 1 and vaccinia virus (VV) infections in normal human keratinocytes (NHKs) and in murine infection models.
Methods: NHKs were treated with S aureus toxins before incubation of viruses. BALB/c mice were inoculated with S aureus 2 days before VV scarification. Viral loads of HSV-1 and VV were evaluated by using real-time PCR, a viral plaque-forming assay, and immunofluorescence staining. Small interfering RNA duplexes were used to knockdown the gene expression of the cellular receptor of α-toxin, a disintegrin and metalloprotease 10 (ADAM10). ADAM10 protein and α-toxin heptamers were detected by using Western blot assays.
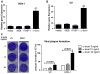
Results: We demonstrate that sublytic staphylococcal α-toxin increases viral loads of HSV-1 and VV in NHKs. Furthermore, we demonstrate in vivo that the VV load is significantly greater (P < .05) in murine skin inoculated with an α-toxin-producing S aureus strain compared with murine skin inoculated with the isogenic α-toxin-deleted strain. The viral enhancing effect of α-toxin is mediated by ADAM10 and is associated with its pore-forming property. Moreover, we demonstrate that α-toxin promotes viral entry in NHKs.
Conclusion: The current study introduces the novel concept that staphylococcal α-toxin promotes viral skin infection and provides a mechanism by which S aureus infection might predispose the host toward disseminated viral infections.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: P. M. Schlievert has received research support from the National Institutes of Health. The rest of the authors declare that they have no relevant conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

