Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy
- PMID: 22841313
- PMCID: PMC3408581
- DOI: 10.1016/j.neuron.2012.05.019
Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy
Abstract
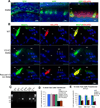
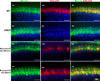
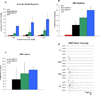
Mice lacking the vesicular glutamate transporter-3 (VGLUT3) are congenitally deaf due to loss of glutamate release at the inner hair cell afferent synapse. Cochlear delivery of VGLUT3 using adeno-associated virus type 1 (AAV1) leads to transgene expression in only inner hair cells (IHCs), despite broader viral uptake. Within 2 weeks of AAV1-VGLUT3 delivery, auditory brainstem response (ABR) thresholds normalize, along with partial rescue of the startle response. Lastly, we demonstrate partial reversal of the morphologic changes seen within the afferent IHC ribbon synapse. These findings represent a successful restoration of hearing by gene replacement in mice, which is a significant advance toward gene therapy of human deafness.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Have you heard? Viral-mediated gene therapy restores hearing.Neuron. 2012 Jul 26;75(2):188-90. doi: 10.1016/j.neuron.2012.06.008. Neuron. 2012. PMID: 22841304 Free PMC article.
References
-
- Baker K, Brough DE, Staecker H. Repair of the vestibular system via adenovector delivery of Atoh1: a potential treatment for balance disorders. Adv Otorhinolaryngol. 2009;66:52–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

