Coding of the reach vector in parietal area 5d
- PMID: 22841318
- PMCID: PMC3408621
- DOI: 10.1016/j.neuron.2012.03.041
Coding of the reach vector in parietal area 5d
Abstract
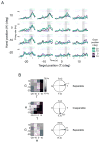
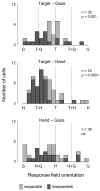
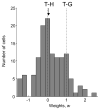
Competing models of sensorimotor computation predict different topological constraints in the brain. Some models propose population coding of particular reference frames in anatomically distinct nodes, whereas others require no such dedicated subpopulations and instead predict that regions will simultaneously code in multiple, intermediate, reference frames. Current empirical evidence is conflicting, partly due to difficulties involved in identifying underlying reference frames. Here, we independently varied the locations of hand, gaze, and target over many positions while recording from the dorsal aspect of parietal area 5. We find that the target is represented in a predominantly hand-centered reference frame here, contrasting with the relative code seen in dorsal premotor cortex and the mostly gaze-centered reference frame in the parietal reach region. This supports the hypothesis that different nodes of the sensorimotor circuit contain distinct and systematic representations, and this constrains the types of computational model that are neurobiologically relevant.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Ann Rev Neurosci. 2002;25:189–220. - PubMed
-
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–583. - PubMed
-
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science. 1985;230:456–458. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

