Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance
- PMID: 22841573
- PMCID: PMC3832894
- DOI: 10.1016/j.cmet.2012.07.002
Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance
Abstract
Obesity-related leptin resistance manifests in loss of leptin's ability to reduce appetite and increase energy expenditure. Obesity is also associated with increased activity of the endocannabinoid system, and CB(1) receptor (CB(1)R) inverse agonists reduce body weight and the associated metabolic complications, although adverse neuropsychiatric effects halted their therapeutic development. Here we show that in mice with diet-induced obesity (DIO), the peripherally restricted CB(1)R inverse agonist JD5037 is equieffective with its brain-penetrant parent compound in reducing appetite, body weight, hepatic steatosis, and insulin resistance, even though it does not occupy central CB(1)R or induce related behaviors. Appetite and weight reduction by JD5037 are mediated by resensitizing DIO mice to endogenous leptin through reversing the hyperleptinemia by decreasing leptin expression and secretion by adipocytes and increasing leptin clearance via the kidney. Thus, inverse agonism at peripheral CB(1)R not only improves cardiometabolic risk in obesity but has antiobesity effects by reversing leptin resistance.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
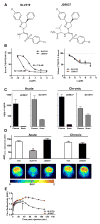


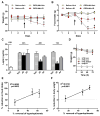


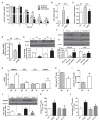
Comment in
-
Obesity: CB1R inverse agonists--antiobesity effects without the neuropsychiatric adverse effects?Nat Rev Endocrinol. 2012 Oct;8(10):564. doi: 10.1038/nrendo.2012.145. Epub 2012 Aug 14. Nat Rev Endocrinol. 2012. PMID: 22890006 No abstract available.
-
Metabolic disorders: Safe cannabinoid receptor modulators in sight?Nat Rev Drug Discov. 2012 Oct;11(10):749. doi: 10.1038/nrd3851. Epub 2012 Sep 24. Nat Rev Drug Discov. 2012. PMID: 23000685 No abstract available.
References
-
- Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohorquez SM, Stoch A, et al. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68–78. - PubMed
-
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. BetaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. - PubMed
-
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. - PubMed
-
- Birn H, Vorum H, Verroust PJ, Moestrup SK, Christensen EI. Receptor-associated protein is important for normal processing of megalin in kidney proximal tubules. J Am Soc Nephrol. 2000;11:191–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

