Limb anterior-posterior polarity integrates activator and repressor functions of GLI2 as well as GLI3
- PMID: 22841643
- PMCID: PMC3432687
- DOI: 10.1016/j.ydbio.2012.07.017
Limb anterior-posterior polarity integrates activator and repressor functions of GLI2 as well as GLI3
Abstract
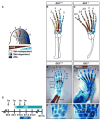
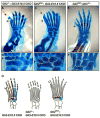
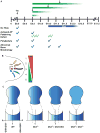
Anterior-posterior (AP) limb patterning is directed by sonic hedgehog (SHH) signaling from the posteriorly located zone of polarizing activity (ZPA). GLI3 and GLI2 are the transcriptional mediators generally utilized in SHH signaling, and each can function as an activator (A) and repressor (R). Although GLI3R has been suggested to be the primary effector of SHH signaling during limb AP patterning, a role for GLI3A or GLI2 has not been fully ruled out, nor has it been determined whether Gli3 plays distinct roles in limb development at different stages. By conditionally removing Gli3 in the limb at multiple different time points, we uncovered four Gli3-mediated functions in limb development that occur at distinct but partially over-lapping time windows: AP patterning of the proximal limb, AP patterning of the distal limb, regulation of digit number and bone differentiation. Furthermore, by removing Gli2 in Gli3 temporal conditional knock-outs, we uncovered an essential role for Gli2 in providing the remaining posterior limb patterning seen in Gli3 single mutants. To test whether GLIAs or GLIRs regulate different aspects of AP limb patterning and/or digit number, we utilized a knock-in allele in which GLI1, which functions solely as an activator, is expressed in place of the bifunctional GLI2 protein. Interestingly, we found that GLIAs contribute to AP patterning specifically in the posterior limb, whereas GLIRs predominantly regulate anterior patterning and digit number. Since GLI3 is a more effective repressor, our results explain why GLI3 is required only for anterior limb patterning and why GLI2 can compensate for GLI3A in posterior limb patterning. Taken together, our data suggest that establishment of a complete range of AP positional identities in the limb requires integration of the spatial distribution, timing, and dosage of GLI2 and GLI3 activators and repressors.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures






References
-
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–16. - PubMed
-
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–72. - PubMed
-
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–15. - PubMed
-
- Barna M, Pandolfi PP, Niswander L. Gli3 and Plzf cooperate in proximal limb patterning at early stages of limb development. Nature. 2005;436:277–81. - PubMed
-
- Bastida MF, Ros MA. How do we get a perfect complement of digits? Curr Opin Genet Dev. 2008;18:374–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

