Regional cell shape changes control form and function of Kupffer's vesicle in the zebrafish embryo
- PMID: 22841644
- PMCID: PMC3586254
- DOI: 10.1016/j.ydbio.2012.07.019
Regional cell shape changes control form and function of Kupffer's vesicle in the zebrafish embryo
Abstract
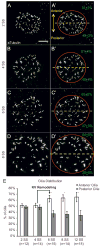
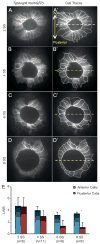
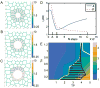
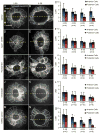
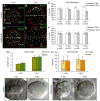
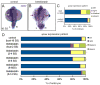
Cilia-generated fluid flow in an 'organ of asymmetry' is critical for establishing the left-right body axis in several vertebrate embryos. However, the cell biology underlying how motile cilia produce coordinated flow and asymmetric signals is not well defined. In the zebrafish organ of asymmetry-called Kupffer's vesicle (KV)-ciliated cells are asymmetrically positioned along the anterior-posterior axis such that more cilia are placed in the anterior region. We previously demonstrated that Rho kinase 2b (Rock2b) is required for anteroposterior asymmetry and fluid flow in KV, but it remained unclear how the distribution of ciliated cells becomes asymmetric during KV development. Here, we identify a morphogenetic process we refer to as 'KV remodeling' that transforms initial symmetry in KV architecture into anteroposterior asymmetry. Live imaging of KV cells revealed region-specific cell shape changes that mediate tight packing of ciliated cells into the anterior pole. Mathematical modeling indicated that different interfacial tensions in anterior and posterior KV cells are involved in KV remodeling. Interfering with non-muscle myosin II (referred to as Myosin II) activity, which modulates cellular interfacial tensions and is regulated by Rock proteins, disrupted KV cell shape changes and the anteroposterior distribution of KV cilia. Similar defects were observed in Rock2b depleted embryos. Furthermore, inhibiting Myosin II at specific stages of KV development perturbed asymmetric flow and left-right asymmetry. These results indicate that regional cell shape changes control the development of anteroposterior asymmetry in KV, which is necessary to generate coordinated asymmetric fluid flow and left-right patterning of the embryo.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer’s vesicle in zebrafish. Dev Biol. 2007;310:196–210. - PubMed
-
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–412. - PubMed
-
- Brakke K. The surface evolver. Exp Math. 1992;1:141–165.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

