Molecular signature and in vivo behavior of bone marrow endosteal and subendosteal stromal cell populations and their relevance to hematopoiesis
- PMID: 22841688
- PMCID: PMC3445703
- DOI: 10.1016/j.yexcr.2012.07.009
Molecular signature and in vivo behavior of bone marrow endosteal and subendosteal stromal cell populations and their relevance to hematopoiesis
Abstract
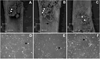
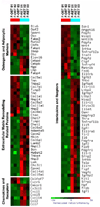
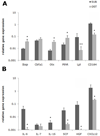
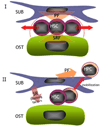
In the bone marrow cavity, hematopoietic stem cells (HSC) have been shown to reside in the endosteal and subendosteal perivascular niches, which play specific roles on HSC maintenance. Although cells with long-term ability to reconstitute full hematopoietic system can be isolated from both niches, several data support a heterogenous distribution regarding the cycling behavior of HSC. Whether this distinct behavior depends upon the role played by the stromal populations which distinctly create these two niches is a question that remains open. In the present report, we used our previously described in vivo assay to demonstrate that endosteal and subendosteal stromal populations are very distinct regarding skeletal lineage differentiation potential. This was further supported by a microarray-based analysis, which also demonstrated that these two stromal populations play distinct, albeit complementary, roles in HSC niche. Both stromal populations were preferentially isolated from the trabecular region and behave distinctly in vitro, as previously reported. Even though these two niches are organized in a very close range, in vivo assays and molecular analyses allowed us to identify endosteal stroma (F-OST) cells as fully committed osteoblasts and subendosteal stroma (F-RET) cells as uncommitted mesenchymal cells mainly represented by perivascular reticular cells expressing high levels of chemokine ligand, CXCL12. Interestingly, a number of cytokines and growth factors including interleukin-6 (IL-6), IL-7, IL-15, Hepatocyte growth factor (HGF) and stem cell factor (SCF) matrix metalloproteases (MMPs) were also found to be differentially expressed by F-OST and F-RET cells. Further microarray analyses indicated important mechanisms used by the two stromal compartments in order to create and coordinate the "quiescent" and "proliferative" niches in which hematopoietic stem cells and progenitors reside.
Published by Elsevier Inc.
Conflict of interest statement
Alex Balduino - designed research; performed research; analyzed data; wrote paper
Valeria Mello Coelho - designed research; analyzed data; wrote paper
Zhou Wang – performed research; analyzed data; wrote paper
Russell S. Taichman – designed research; analyzed data; wrote paper
Paul H. Krebsbach – analyzed data; wrote paper
Ashani T. Weeraratna - designed research; performed research; analyzed data
Kevin G. Becker - designed research; performed research; analyzed data
Wallace de Mello - performed research; analyzed
Dennis D. Taub - designed research; analyzed data; wrote paper
Radovan Borojevic - designed research; analyzed data; wrote paper
The authors declare no competing financial or potential conflict of interests.
Figures


Similar articles
-
Bone marrow subendosteal microenvironment harbours functionally distinct haemosupportive stromal cell populations.Cell Tissue Res. 2005 Feb;319(2):255-66. doi: 10.1007/s00441-004-1006-3. Epub 2004 Dec 1. Cell Tissue Res. 2005. PMID: 15578225
-
Regulation of hematopoietic stem cells by bone marrow stromal cells.Trends Immunol. 2014 Jan;35(1):32-7. doi: 10.1016/j.it.2013.10.002. Epub 2013 Nov 5. Trends Immunol. 2014. PMID: 24210164 Free PMC article. Review.
-
CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance.Nature. 2013 Mar 14;495(7440):227-30. doi: 10.1038/nature11926. Epub 2013 Feb 24. Nature. 2013. PMID: 23434756 Free PMC article.
-
Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells.Proc Natl Acad Sci U S A. 2013 Jul 30;110(31):12643-8. doi: 10.1073/pnas.1310212110. Epub 2013 Jul 15. Proc Natl Acad Sci U S A. 2013. PMID: 23858471 Free PMC article.
-
[Bone and Stem Cells. Bone marrow microenvironment niches for hematopoietic stem and progenitor cells].Clin Calcium. 2014 Apr;24(4):517-26. Clin Calcium. 2014. PMID: 24681497 Review. Japanese.
Cited by
-
CXCR4 mediates the effects of IGF-1R signaling in rodent bone homeostasis and fracture repair.Bone. 2023 Jan;166:116600. doi: 10.1016/j.bone.2022.116600. Epub 2022 Nov 9. Bone. 2023. PMID: 36368465 Free PMC article.
-
Where is the common ground between bone marrow mesenchymal stem/stromal cells from different donors and species?Stem Cell Res Ther. 2015 Aug 18;6(1):143. doi: 10.1186/s13287-015-0144-8. Stem Cell Res Ther. 2015. PMID: 26282627 Free PMC article. Review.
-
Adipose stromal/stem cells in regenerative medicine: Potentials and limitations.World J Stem Cells. 2020 Jan 26;12(1):1-7. doi: 10.4252/wjsc.v12.i1.1. World J Stem Cells. 2020. PMID: 32110271 Free PMC article.
-
The bone marrow endosteal niche: how far from the surface?J Cell Biochem. 2015 Jan;116(1):6-11. doi: 10.1002/jcb.24952. J Cell Biochem. 2015. PMID: 25164953 Free PMC article.
-
Role of Prx1-expressing skeletal cells and Prx1-expression in fracture repair.Bone. 2020 Oct;139:115521. doi: 10.1016/j.bone.2020.115521. Epub 2020 Jul 3. Bone. 2020. PMID: 32629173 Free PMC article.
References
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- Balduino A, Hurtado SP, Frazão P, Takiya CM, Alves LM, Nasciutti LE, El-Cheikh MC, Borojevic R. Bone marrow subendosteal microenvironment harbours functionally distinct haemosupportive stromal cell populations. Cell Tissue Res. 2005;319:255–266. - PubMed
-
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. - PubMed
-
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID, Rafii S. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

