Crypt base columnar stem cells in small intestines of mice are radioresistant
- PMID: 22841781
- PMCID: PMC3480544
- DOI: 10.1053/j.gastro.2012.07.106
Crypt base columnar stem cells in small intestines of mice are radioresistant
Abstract
Background & aims: Adult stem cells have been proposed to be quiescent and radiation resistant, repairing DNA double-strand breaks by nonhomologous end joining. However, the population of putative small intestinal stem cells (ISCs) at position +4 from the crypt base contradicts this model, in that they are highly radiosensitive. Cycling crypt base columnar cells (CBCs) at crypt positions +1-3 recently were defined as an alternative population of ISCs. Little is known about the sensitivity of this stem cell population to radiation.
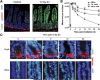
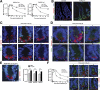
Methods: Radiation-induced lethality of CBCs was quantified kinetically in Lgr5-lacZ transgenic mice. γ-H2AX, BRCA1, RAD51, and DNA-PKcs foci were used as DNA repair surrogates to investigate the inherent ability of CBCs to recognize and repair double-strand breaks. 5-ethynyl-2'-deoxyuridine and 5-bromo-2'-deoxyuridine incorporation assays were used to study patterns of CBC growth arrest and re-initiation of cell cycling. Apoptosis was evaluated by caspase-3 staining.
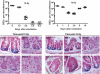
Results: CBCs are relatively radioresistant, repairing DNA by homologous recombination significantly more efficiently than transit amplifying progenitors or villus cells. CBCs undergo apoptosis less than 24 hours after irradiation (32% ± 2% of total lethality) or mitotic death at 24-48 hours. Survival of CBCs at 2 days predicts crypt regeneration at 3.5 days and lethality from gastrointestinal syndrome. Crypt repopulation originates from CBCs that survive irradiation.
Conclusions: Adult ISCs in mice can cycle rapidly yet still be radioresistant. Importantly, homologous recombination can protect adult stem cell populations from genotoxic stress. These findings broaden and refine concepts of the phenotype of adult stem cells.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

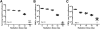

Comment in
-
Fixing the breaks in intestinal stem cells after radiation: a matter of DNA damage and death or DNA repair and regeneration.Gastroenterology. 2012 Nov;143(5):1144-1147. doi: 10.1053/j.gastro.2012.09.021. Epub 2012 Sep 19. Gastroenterology. 2012. PMID: 23000480 No abstract available.
References
-
- Sotiropoulou PA, Candi A, Mascre G, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572–582. - PubMed
-
- Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–136. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

