The constant region contributes to the antigenic specificity and renal pathogenicity of murine anti-DNA antibodies
- PMID: 22841793
- PMCID: PMC3656415
- DOI: 10.1016/j.jaut.2012.06.005
The constant region contributes to the antigenic specificity and renal pathogenicity of murine anti-DNA antibodies
Abstract
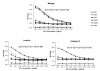
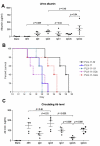
Affinity for DNA and cross-reactivity with renal antigens are associated with enhanced renal pathogenicity of lupus autoantibodies. In addition, certain IgG subclasses are enriched in nephritic kidneys, suggesting that isotype may determine the outcome of antibody binding to renal antigens. To investigate if the isotype of DNA antibodies affects renal pathogenicity by influencing antigen binding, we derived IgM, IgG1, IgG2b and IgG2a forms of the PL9-11 antibody (IgG3 anti-DNA) by in vitro class switching or PCR cloning. The affinity and specificity of PL9-11 antibodies for nuclear and renal antigens were analyzed using ELISA, Western blotting, surface plasmon resonance (SPR), binding to mesangial cells, and glomerular proteome arrays. Renal deposition and pathogenicity were assayed in mice injected with PL9-11 hybridomas. We found that PL9-11 and its isotype-switched variants had differential binding to DNA and chromatin (IgG3>IgG2a>IgG1>IgG2b>IgM) by direct and competition ELISA, and SPR. In contrast, in binding to laminin and collagen IV the IgG2a isotype actually had the highest affinity. Differences in affinity of PL9-11 antibodies for renal antigens were mirrored in analysis of specificity for glomeruli, and were associated with significant differences in renal pathogenicity in vivo and survival. Our novel findings indicate that the constant region plays an important role in the nephritogenicity of antibodies to DNA by affecting immunoglobulin affinity and specificity. Increased binding to multiple glomerular and/or nuclear antigens may contribute to the renal pathogenicity of anti-DNA antibodies of the IgG2a and IgG3 isotype. Finally, class switch recombination may be another mechanism by which B cell autoreactivity is generated.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures






References
-
- Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Sem Arthritis Rheum. 2004;34:501–37. - PubMed
-
- Jang EJ, Nahm DH, Jang YJ. Mouse monoclonal autoantibodies penetrate mouse macrophage cells and stimulate NF-kappaB activation and TNF-alpha release. Immunology Lett. 2009;124:70–6. - PubMed
-
- Yung S, Tsang RC, Sun Y, Leung JK, Chan TM. Effect of human anti-DNA antibodies on proximal renal tubular epithelial cell cytokine expression: implications on tubulointerstitial inflammation in lupus nephritis. J Amer Soc Nephrol. 2005;16:3281–94. - PubMed
-
- Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI033142/AI/NIAID NIH HHS/United States
- R01 AI033774/AI/NIAID NIH HHS/United States
- DK090319/DK/NIDDK NIH HHS/United States
- R01 HL059842/HL/NHLBI NIH HHS/United States
- HL059842/HL/NHLBI NIH HHS/United States
- R01 AI052733/AI/NIAID NIH HHS/United States
- AI052733/AI/NIAID NIH HHS/United States
- R56 AR048692/AR/NIAMS NIH HHS/United States
- R37 AI033142/AI/NIAID NIH HHS/United States
- R01 AR048692/AR/NIAMS NIH HHS/United States
- AI033142/AI/NIAID NIH HHS/United States
- AI033774/AI/NIAID NIH HHS/United States
- R01 DK090319/DK/NIDDK NIH HHS/United States
- AR048692/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

