Nanomolar ouabain increases NCX1 expression and enhances Ca2+ signaling in human arterial myocytes: a mechanism that links salt to increased vascular resistance?
- PMID: 22842068
- PMCID: PMC3469703
- DOI: 10.1152/ajpheart.00399.2012
Nanomolar ouabain increases NCX1 expression and enhances Ca2+ signaling in human arterial myocytes: a mechanism that links salt to increased vascular resistance?
Abstract
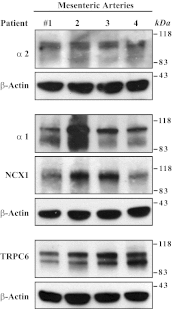
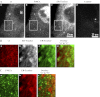
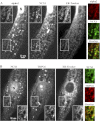
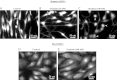
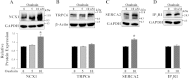
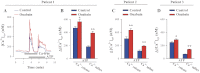
The mechanisms by which NaCl raises blood pressure (BP) in hypertension are unresolved, but much evidence indicates that endogenous ouabain is involved. In rodents, arterial smooth muscle cell (ASMC) Na(+) pumps with an α(2)-catalytic subunit (ouabain EC(50) ≤1.0 nM) are crucial for some hypertension models, even though ≈80% of ASMC Na(+) pumps have an α(1)-subunit (ouabain EC(50) ≈ 5 μM). Human α(1)-Na(+) pumps, however, have high ouabain affinity (EC(50) ≈ 10-20 nM). We used immunoblotting, immunocytochemistry, and Ca(2+) imaging (fura-2) to examine the expression, distribution, and function of Na(+) pump α-subunit isoforms in human arteries and primary cultured human ASMCs (hASMCs). hASMCs express α(1)- and α(2)-Na(+) pumps. Further, α(2)-, but not α(1)-, pumps are confined to plasma membrane microdomains adjacent to sarcoplasmic reticulum (SR), where they colocalize with Na/Ca exchanger-1 (NCX1) and C-type transient receptor potential-6 (receptor-operated channels, ROCs). Prolonged inhibition (72 h) with 100 nM ouabain (blocks nearly all α(1)- and α(2)-pumps) was toxic to most cultured hASMCs. Treatment with 10 nM ouabain (72 h), however, increased NCX1 and sarco(endo)plasmic reticulum Ca(2+)-ATPase expression and augmented ATP (10 μM)-induced SR Ca(2+) release in 0 Ca(2+), ouabain-free media, and Ca(2+) influx after external Ca(2+) restoration. The latter was likely mediated primarily by ROCs and store-operated Ca(2+) channels. These hASMC protein expression and Ca(2+) signaling changes are comparable with previous observations on myocytes isolated from arteries of many rat hypertension models. We conclude that the same structurally and functionally coupled mechanisms (α(2)-Na(+) pumps, NCX1, ROCs, and the SR) regulate Ca(2+) homeostasis and signaling in hASMCs and rodent ASMCs. These ouabain/endogenous ouabain-modulated mechanisms underlie the whole body autoregulation associated with increased vascular resistance and elevation of BP in human, salt-sensitive hypertension.
Figures

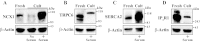

References
-
- Bae YM, Kim A, Lee YJ, Lim W, Noh YH, Kim EJ, Kim J, Kim TK, Park SW, Kim B, Cho SI, Kim DK, Ho WK. Enhancement of receptor-operated cation current and TRPC6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 25: 809–817, 2007 - PubMed
-
- Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297: C1103–C1112, 2009 - PMC - PubMed
-
- Berra-Romani R, Blaustein MP, Matteson DR. TTX-sensitive voltage-gated Na+ channels are expressed in mesenteric artery smooth muscle cells. Am J Physiol Heart Circ Physiol 289: H137–H145, 2005 - PubMed
-
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

