Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity
- PMID: 22842072
- PMCID: PMC3427462
- DOI: 10.1016/j.neuropharm.2012.07.034
Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity
Abstract
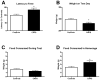
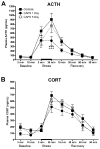
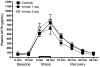
Exposure to psychological trauma is the precipitating factor for PTSD. In addition, a history of chronic or traumatic stress exposure is a predisposing risk factor. We have developed a Chronic plus Acute Prolonged Stress (CAPS) treatment for rats that models some of the characteristics of stressful events that can lead to PTSD in humans. We have previously shown that CAPS enhances acute fear responses and impairs extinction of conditioned fear. Further, CAPS reduced the expression of glucocorticoid receptors in the medial prefrontal cortex. In this study we examined the effects of CAPS exposure on behavioral stress coping style, anxiety-like behaviors, and acute stress reactivity of the hypothalamic-pituitary-adrenal (HPA) axis. Male Sprague-Dawley rats were exposed to CAPS treatment, consisting of chronic intermittent cold stress (4 °C, 6 h/day, 14 days) followed on day 15 by a single 1-h session of sequential acute stressors (social defeat, immobilization, swim). After CAPS or control treatment, different groups were tested for shock probe defensive burying, novelty suppressed feeding, or evoked activation of adrenocorticotropic hormone (ACTH) and corticosterone release by an acute immobilization stress. CAPS resulted in a decrease in active burying behavior and an increase in immobility in the shock probe test. Further, CAPS-treated rats displayed increases in the latency to feed in the novelty suppressed feeding test, despite an increase in food intake in the home cage. CAPS treatment also reduced the HPA response to a subsequent acute immobilization stress. These results further validate CAPS treatment as a rat model of relevance to PTSD, and together with results reported previously, suggest that CAPS impairs fear extinction, shifts coping behavior from an active to a more passive strategy, increases anxiety, and alters HPA reactivity, resembling many aspects of human PTSD.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C: 2000. text rev.
-
- Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. - PubMed
-
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioral responses. Behav Res Ther. 2007;45:2019–2033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

