Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan
- PMID: 22842218
- PMCID: PMC3469632
- DOI: 10.1152/ajplung.00406.2011
Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan
Abstract
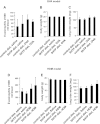
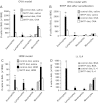
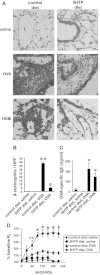
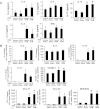
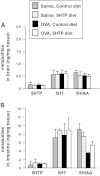
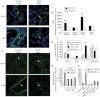
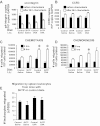
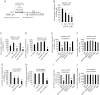
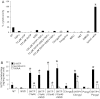
Clinical reports indicate that patients with allergy/asthma commonly have associated symptoms of anxiety/depression. Anxiety/depression can be reduced by 5-hydroxytryptophan (5-HTP) supplementation. However, it is not known whether 5-HTP reduces allergic inflammation. Therefore, we determined whether 5-HTP supplementation reduces allergic inflammation. We also determined whether 5-HTP decreases passage of leukocytes through the endothelial barrier by regulating endothelial cell function. For these studies, C57BL/6 mice were supplemented with 5-HTP, treated with ovalbumin fraction V (OVA), house dust mite (HDM) extract, or IL-4, and examined for allergic lung inflammation and OVA-induced airway responsiveness. To determine whether 5-HTP reduces leukocyte or eosinophil transendothelial migration, endothelial cells were pretreated with 5-HTP, washed and then used in an in vitro transendothelial migration assay under laminar flow. Interestingly, 5-HTP reduced allergic lung inflammation by 70-90% and reduced antigen-induced airway responsiveness without affecting body weight, blood eosinophils, cytokines, or chemokines. 5-HTP reduced allergen-induced transglutaminase 2 (TG2) expression and serotonylation (serotonin conjugation to proteins) in lung endothelial cells. Consistent with the regulation of endothelial serotonylation in vivo, in vitro pretreatment of endothelial cells with 5-HTP reduced TNF-α-induced endothelial cell serotonylation and reduced leukocyte transendothelial migration. Furthermore, eosinophil and leukocyte transendothelial migration was reduced by inhibitors of transglutaminase and by inhibition of endothelial cell serotonin synthesis, suggesting that endothelial cell serotonylation is key for leukocyte transendothelial migration. In summary, 5-HTP supplementation inhibits endothelial serotonylation, leukocyte recruitment, and allergic inflammation. These data identify novel potential targets for intervention in allergy/asthma.
Figures





References
-
- Abdouh M, Albert PR, Drobetsky E, Filep JG, Kouassi E. 5-HT1A-mediated promotion of mitogen-activated T and B cell survival and proliferation is associated with increased translocation of NF-kappaB to the nucleus. Brain Behav Immun 18: 24–34, 2004 - PubMed
-
- Abdouh M, Storring JM, Riad M, Paquette Y, Albert PR, Drobetsky E, Kouassi E. Transcriptional mechanisms for induction of 5-HT1A receptor mRNA and protein in activated B and T lymphocytes. J Biol Chem 276: 4382–4388, 2001 - PubMed
-
- Alberghina D, Amorini AM, Lazzarino G. Modulation of peripheral markers of the serotoninergic system in healthy horses. Res Vet Sci 90: 392–395, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

