Regulation of Rela/p65 and endothelial cell inflammation by proline-rich tyrosine kinase 2
- PMID: 22842493
- PMCID: PMC3547104
- DOI: 10.1165/rcmb.2012-0047OC
Regulation of Rela/p65 and endothelial cell inflammation by proline-rich tyrosine kinase 2
Abstract
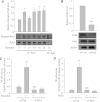
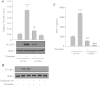
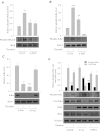
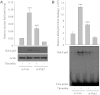
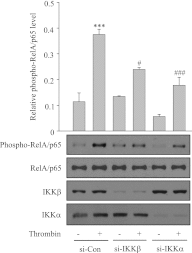
We investigated the role of proline-rich tyrosine kinase 2 (Pyk2) in the mechanism of NF-κB activation and endothelial cell (EC) inflammation induced by thrombin, a procoagulant serine protease released in high amounts during sepsis and other inflammatory conditions. Stimulation of ECs with thrombin resulted in a time-dependent activation of Pyk2. RNA interference knockdown of Pyk2 attenuated thrombin-induced activity of NF-κB and expression of its target genes, vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1. Pyk2 knockdown impaired thrombin-induced activation of IκB kinase (IKK) and phosphorylation (Ser32 and Ser36) of IkappaBα, but, surprisingly, failed to prevent IκBα degradation. However, depletion of IKKα or IKKβ was effective in inhibiting IκBα phosphorylation/degradation, as expected. Intriguingly, Pyk2 knockdown impaired nuclear translocation and DNA binding of RelA/p65, despite the inability to prevent IκBα degradation. In addition, Pyk2 knockdown was associated with inhibition of RelA/p65 phosphorylation at Ser536, which is important for transcriptional activity of RelA/p65. Depletion of IKKα or IKKβ each impaired RelA/p65 phosphorylation. Taken together, these data identify Pyk2 as a critical regulator of EC inflammation by virtue of engaging IKK to promote the release and the transcriptional capacity of RelA/p65, and, additionally, by its ability to facilitate the nuclear translocation of the released RelA/p65. Thus, specific targeting of Pyk2 may be an effective anti-inflammatory strategy in vascular diseases associated with EC inflammation and intravascular coagulation.
Figures
References
-
- Ghosh S, Hayden MS. New regulators of NF-kappa B in inflammation. Nat Rev Immunol 2008;8:837–848 - PubMed
-
- Bijli KM, Rahman ANF. NF-κB signaling in endothelium. In: Aird WC, editor. Endothelial biomedicine. New York: Cambridge University Press; 2007. pp. 784–795.
-
- Karin M. Nuclear factor-kappa B in cancer development and progression. Nature 2006;441:431–436 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

