Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization
- PMID: 22842495
- PMCID: PMC3547103
- DOI: 10.1165/rcmb.2012-0161OC
Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization
Abstract
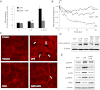
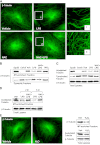
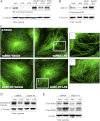
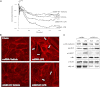
Oxidative stress is an important part of host innate immune response to foreign pathogens, such as bacterial LPS, but excessive activation of redox signaling may lead to pathologic endothelial cell (EC) activation and barrier dysfunction. Microtubules (MTs) play an important role in agonist-induced regulation of vascular endothelial permeability, but their impact in modulation of inflammation and EC barrier has not been yet investigated. This study examined the effects of LPS-induced oxidative stress on MT dynamics and the involvement of MTs in the LPS-induced mechanisms of Rho activation, EC permeability, and lung injury. LPS treatment of pulmonary vascular EC induced elevation of reactive oxygen species (ROS) and caused oxidative stress associated with EC hyperpermeability, cytoskeletal remodeling, and formation of paracellular gaps, as well as activation of Rho, p38 stress kinase, and NF-κB signaling, the hallmarks of endothelial barrier dysfunction. LPS also triggered ROS-dependent disassembly of the MT network, leading to activation of MT-dependent signaling. Stabilization of MTs with epothilone B, or inhibition of MT-associated guanine nucleotide exchange factor (GEF)-H1 activity by silencing RNA-mediated knockdown, suppressed LPS-induced EC barrier dysfunction in vitro, and attenuated vascular leak and lung inflammation in vivo. LPS disruptive effects were linked to activation of Rho signaling caused by LPS-induced MT disassembly and release of Rho-specific GEF-H1 from MTs. These studies demonstrate, for the first time, the mechanism of ROS-induced Rho activation via destabilization of MTs and GEF-H1-dependent activation of Rho signaling, leading to pulmonary EC barrier dysfunction and exacerbation of LPS-induced inflammation.
Figures



References
-
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006;86:279–367 - PubMed
-
- Dosquet C, Weill D, Wautier JL. Molecular mechanism of blood monocyte adhesion to vascular endothelial cells. Nouv Rev Fr Hematol 1992;34:S55–S59 - PubMed
-
- Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil–endothelial cell adhesion. Antioxid Redox Signal 2002;4:39–47 - PubMed
-
- Smith CW. Leukocyte–endothelial cell interactions. Semin Hematol 1993;30(4 Suppl 4):45–53; discussion 54–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

