ROS homeostasis during development: an evolutionary conserved strategy
- PMID: 22842779
- PMCID: PMC11114851
- DOI: 10.1007/s00018-012-1092-4
ROS homeostasis during development: an evolutionary conserved strategy
Abstract
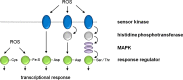
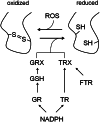
The balance between cellular proliferation and differentiation is a key aspect of development in multicellular organisms. Recent studies on Arabidopsis roots revealed distinct roles for different reactive oxygen species (ROS) in these processes. Modulation of the balance between ROS in proliferating cells and elongating cells is controlled at least in part at the transcriptional level. The effect of ROS on proliferation and differentiation is not specific for plants but appears to be conserved between prokaryotic and eukaryotic life forms. The ways in which ROS is received and how it affects cellular functioning is discussed from an evolutionary point of view. The different redox-sensing mechanisms that evolved ultimately result in the activation of gene regulatory networks that control cellular fate and decision-making. This review highlights the potential common origin of ROS sensing, indicating that organisms evolved similar strategies for utilizing ROS during development, and discusses ROS as an ancient universal developmental regulator.
Figures

References
-
- Falkowski PG. Evolution: tracing oxygen’s imprint on earth’s metabolic evolution. Science. 2006;311:1724–1725. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

