Trafficking of PrPc to mitochondrial raft-like microdomains during cell apoptosis
- PMID: 22842913
- PMCID: PMC3609063
- DOI: 10.4161/pri.20479
Trafficking of PrPc to mitochondrial raft-like microdomains during cell apoptosis
Abstract
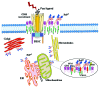
The cellular form of prion protein (PrP (c)) is a highly conserved cell surface GPI-anchored glycoprotein that was identified in cholesterol-enriched, detergent-resistant microdomains, named "rafts." The association with these specialized portions of the cell plasma membrane is required for conversion of PrP (c) to the transmissible spongiform encephalopathy-associated protease-resistant isoform. Usually, PrP (c) is reported to be a plasma membrane protein, however several studies have revealed PrP (c) as an interacting protein mainly with the membrane/organelles, as well as with cytoskeleton network. Recent lines of evidence indicated its association with ER lipid raft-like microdomains for a correct folding of PrP (c), as well as for the export of the protein to the Golgi and proper glycosylation. During cell apoptosis, PrP (c) can undergo intracellular re-localization, via ER-mitochondria associated membranes (MAM) and microtubular network, to mitochondrial raft-like microdomains, where it induced the loss of mitochondrial membrane potential and citochrome c release, after a contained raise of calcium concentration. We suggest that PrP (c) may play a role in the multimolecular signaling complex associated with cell apoptosis Lipid rafts and their components may, thus, be investigated as pharmacological targets of interest, introducing a novel and innovative task in modern pharmacology, i.e., the development of glycosphingolipid targeted drugs.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
