Six commercially available angiotensin II AT1 receptor antibodies are non-specific
- PMID: 22843099
- PMCID: PMC3508356
- DOI: 10.1007/s10571-012-9862-y
Six commercially available angiotensin II AT1 receptor antibodies are non-specific
Abstract
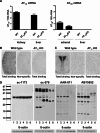
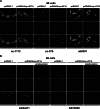
Commercially available Angiotensin II AT1 receptor antibodies are widely employed for receptor localization and quantification, but they have not been adequately validated. In this study, six commercially available AT1 receptor antibodies were characterized by established criteria: sc-1173 and sc-579 from Santa Cruz Biotechnology, Inc., AAR-011 from Alomone Labs, Ltd., AB15552 from Millipore, and ab18801 and ab9391 from Abcam. The immunostaining patterns observed were different for every antibody tested, and were unrelated to the presence or absence of AT1 receptors. The antibodies detected a 43 kDa band in western blots, corresponding to the predicted size of the native AT1 receptor. However, identical bands were observed in wild-type mice and in AT1A knock-out mice not expressing the target protein. Moreover, immunoreactivity detected in rat hypothalamic 4B cells not expressing AT1 receptors or transfected with AT1A receptor construct was identical, as revealed by western blotting and immunocytochemistry in cultured 4B cells. Additional prominent immunoreactive bands above and below 43 kDa were observed by western blotting in extracts from tissues of AT1A knock-out and wild-type mice and in 4B cells with or without AT1 receptor expression. In all cases, the patterns of immunoreactivity were independent of the AT1 receptor expression and different for each antibody studied. We conclude that, in our experimental setup, none of the commercially available AT1 receptor antibodies tested met the criteria for specificity and that competitive radioligand binding remains the only reliable approach to study AT1 receptor physiology in the absence of full antibody characterization.
Figures


References
-
- Burson JM, Aguilera G, Gross KW, Sigmund CD (1994) Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol 267:E260–E267 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

