Mitochondrial metabolism in Parkinson's disease impairs quality control autophagy by hampering microtubule-dependent traffic
- PMID: 22843496
- PMCID: PMC3471400
- DOI: 10.1093/hmg/dds309
Mitochondrial metabolism in Parkinson's disease impairs quality control autophagy by hampering microtubule-dependent traffic
Abstract
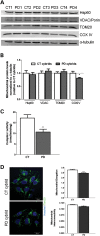
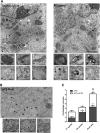
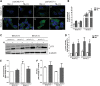
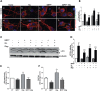
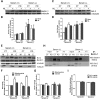
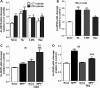
Abnormal presence of autophagic vacuoles is evident in brains of patients with Parkinson's disease (PD), in contrast to the rare detection of autophagosomes in a normal brain. However, the actual cause and pathological significance of these observations remain unknown. Here, we demonstrate a role for mitochondrial metabolism in the regulation of the autophagy-lysosomal pathway in ex vivo and in vitro models of PD. We show that transferring mitochondria from PD patients into cells previously depleted of mitochondrial DNA is sufficient to reproduce the alterations in the autophagic system observed in PD patient brains. Although the initial steps of this pathway are not compromised, there is an increased accumulation of autophagosomes associated with a defective autophagic activity. We prove that this functional decline was originated from a deficient mobilization of autophagosomes from their site of formation toward lysosomes due to disruption in microtubule-dependent trafficking. This contributed directly to a decreased proteolytic flux of α-synuclein and other autophagic substrates. Our results lend strong support for a direct impact of mitochondria in autophagy as defective autophagic clearance ability secondary to impaired microtubule trafficking is driven by dysfunctional mitochondria. We uncover mitochondria and mitochondria-dependent intracellular traffic as main players in the regulation of autophagy in PD.
Figures





Similar articles
-
Mitochondrial Metabolism Regulates Microtubule Acetylome and Autophagy Trough Sirtuin-2: Impact for Parkinson's Disease.Mol Neurobiol. 2018 Feb;55(2):1440-1462. doi: 10.1007/s12035-017-0420-y. Epub 2017 Feb 6. Mol Neurobiol. 2018. PMID: 28168426
-
The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson's disease.Biochim Biophys Acta. 2014 Jan;1842(1):7-21. doi: 10.1016/j.bbadis.2013.10.003. Epub 2013 Oct 11. Biochim Biophys Acta. 2014. PMID: 24120997
-
Mitochondria drive autophagy pathology via microtubule disassembly: a new hypothesis for Parkinson disease.Autophagy. 2013 Jan;9(1):112-4. doi: 10.4161/auto.22443. Epub 2012 Oct 17. Autophagy. 2013. PMID: 23075854 Free PMC article.
-
Mitochondrial metabolic control of microtubule dynamics impairs the autophagic pathway in Parkinson's disease.Neurodegener Dis. 2012;10(1-4):38-40. doi: 10.1159/000332601. Epub 2011 Dec 9. Neurodegener Dis. 2012. PMID: 22156537 Review.
-
Mitochondrial function and autophagy: integrating proteotoxic, redox, and metabolic stress in Parkinson's disease.J Neurochem. 2018 Mar;144(6):691-709. doi: 10.1111/jnc.14308. Epub 2018 Feb 14. J Neurochem. 2018. PMID: 29341130 Free PMC article. Review.
Cited by
-
The Role of Bacteria-Mitochondria Communication in the Activation of Neuronal Innate Immunity: Implications to Parkinson's Disease.Int J Mol Sci. 2023 Feb 22;24(5):4339. doi: 10.3390/ijms24054339. Int J Mol Sci. 2023. PMID: 36901773 Free PMC article.
-
Autophagy Stimulation Decreases Dopaminergic Neuronal Death Mediated by Oxidative Stress.Mol Neurobiol. 2019 Dec;56(12):8136-8156. doi: 10.1007/s12035-019-01654-1. Epub 2019 Jun 13. Mol Neurobiol. 2019. PMID: 31197654
-
Mitochondrial Metabolism Regulates Microtubule Acetylome and Autophagy Trough Sirtuin-2: Impact for Parkinson's Disease.Mol Neurobiol. 2018 Feb;55(2):1440-1462. doi: 10.1007/s12035-017-0420-y. Epub 2017 Feb 6. Mol Neurobiol. 2018. PMID: 28168426
-
Comparative Microarray Analysis Identifies Commonalities in Neuronal Injury: Evidence for Oxidative Stress, Dysfunction of Calcium Signalling, and Inhibition of Autophagy-Lysosomal Pathway.Neurochem Res. 2016 Mar;41(3):554-67. doi: 10.1007/s11064-015-1666-2. Epub 2015 Aug 29. Neurochem Res. 2016. PMID: 26318862 Review.
-
A cybrid cell model for the assessment of the link between mitochondrial deficits and sporadic Parkinson's disease.Methods Mol Biol. 2015;1265:415-24. doi: 10.1007/978-1-4939-2288-8_31. Methods Mol Biol. 2015. PMID: 25634293 Free PMC article.
References
-
- Forno L.S. Neuropathology of Parkinson's disease. J. Neuropathol. Exp. Neurol. 1996;55:259–272. - PubMed
-
- Schapira A.H., Cooper J.M., Dexter D., Jenner P., Clark J.B., Marsden C.D. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. - PubMed
-
- Parker W.D., Jr, Boyson S.J., Parks J.K. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 1989;26:719–723. - PubMed
-
- Swerdlow R.H., Parks J.K., Miller S.W., Tuttle J.B., Trimmer P.A., Sheehan J.P., Bennett J.P., Jr, Davis R.E., Parker W.D., Jr Origin and functional consequences of the complex I defect in Parkinson's disease. Ann. Neurol. 1996;40:663–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

