Uncoupling of substrate-level phosphorylation in Escherichia coli during glucose-limited growth
- PMID: 22843529
- PMCID: PMC3457515
- DOI: 10.1128/AEM.01507-12
Uncoupling of substrate-level phosphorylation in Escherichia coli during glucose-limited growth
Abstract
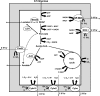
The respiratory chain of Escherichia coli contains three different cytochrome oxidases. Whereas the cytochrome bo oxidase and the cytochrome bd-I oxidase are well characterized and have been shown to contribute to proton translocation, physiological data suggested a nonelectrogenic functioning of the cytochrome bd-II oxidase. Recently, however, this view was challenged by an in vitro biochemical analysis that showed that the activity of cytochrome bd-II oxidase does contribute to proton translocation with an H(+)/e(-) stoichiometry of 1. Here, we propose that this apparent discrepancy is due to the activities of two alternative catabolic pathways: the pyruvate oxidase pathway for acetate production and a pathway with methylglyoxal as an intermediate for the production of lactate. The ATP yields of these pathways are lower than those of the pathways that have so far always been assumed to catalyze the main catabolic flux under energy-limited growth conditions (i.e., pyruvate dehydrogenase and lactate dehydrogenase). Inclusion of these alternative pathways in the flux analysis of growing E. coli strains for the calculation of the catabolic ATP synthesis rate indicates an electrogenic function of the cytochrome bd-II oxidase, compatible with an H(+)/e(-) ratio of 1. This analysis shows for the first time the extent of bypassing of substrate-level phosphorylation in E. coli under energy-limited growth conditions.
Figures
References
-
- Abdel-Hamid AM, Attwood MM, Guest JR. 2001. Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli. Microbiology 147: 1483– 1498 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

