Molecular and biochemical analyses of the GH44 module of CbMan5B/Cel44A, a bifunctional enzyme from the hyperthermophilic bacterium Caldicellulosiruptor bescii
- PMID: 22843537
- PMCID: PMC3457505
- DOI: 10.1128/AEM.02009-12
Molecular and biochemical analyses of the GH44 module of CbMan5B/Cel44A, a bifunctional enzyme from the hyperthermophilic bacterium Caldicellulosiruptor bescii
Abstract
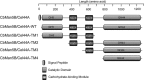
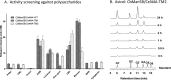
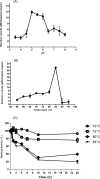
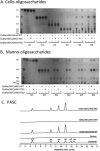
A large polypeptide encoded in the genome of the thermophilic bacterium Caldicellulosiruptor bescii was determined to consist of two glycoside hydrolase (GH) modules separated by two carbohydrate-binding modules (CBMs). Based on the detection of mannanase and endoglucanase activities in the N-terminal GH5 and the C-terminal GH44 module, respectively, the protein was designated CbMan5B/Cel44A. A GH5 module with >99% identity from the same organism was characterized previously (X. Su, R. I. Mackie, and I. K. Cann, Appl. Environ. Microbiol. 78:2230-2240, 2012); therefore, attention was focused on CbMan5A/Cel44A-TM2 (or TM2), which harbors the GH44 module and the two CBMs. On cellulosic substrates, TM2 had an optimal temperature and pH of 85°C and 5.0, respectively. Although the amino acid sequence of the GH44 module of TM2 was similar to those of other GH44 modules that hydrolyzed cello-oligosaccharides, cellulose, lichenan, and xyloglucan, it was unique that TM2 also displayed modest activity on mannose-configured substrates and xylan. The TM2 protein also degraded Avicel with higher specific activity than activities reported for its homologs. The GH44 catalytic module is composed of a TIM-like domain and a β-sandwich domain, which consists of one β-sheet at the N terminus and nine β-sheets at the C terminus. Deletion of one or more β-sheets from the β-sandwich domain resulted in insoluble proteins, suggesting that the β-sandwich domain is essential for proper folding of the polypeptide. Combining TM2 with three other endoglucanases from C. bescii led to modest synergistic activities during degradation of cellulose, and based on our results, we propose a model for cellulose hydrolysis and utilization by C. bescii.
Figures


Similar articles
-
Biochemical and mutational analyses of a multidomain cellulase/mannanase from Caldicellulosiruptor bescii.Appl Environ Microbiol. 2012 Apr;78(7):2230-40. doi: 10.1128/AEM.06814-11. Epub 2012 Jan 13. Appl Environ Microbiol. 2012. PMID: 22247178 Free PMC article.
-
Molecular and biochemical analyses of CbCel9A/Cel48A, a highly secreted multi-modular cellulase by Caldicellulosiruptor bescii during growth on crystalline cellulose.PLoS One. 2013 Dec 16;8(12):e84172. doi: 10.1371/journal.pone.0084172. eCollection 2013. PLoS One. 2013. PMID: 24358340 Free PMC article.
-
Identification of the C-Terminal GH5 Domain from CbCel9B/Man5A as the First Glycoside Hydrolase with Thermal Activation Property from a Multimodular Bifunctional Enzyme.PLoS One. 2016 Jun 3;11(6):e0156802. doi: 10.1371/journal.pone.0156802. eCollection 2016. PLoS One. 2016. PMID: 27258548 Free PMC article.
-
Comparative analyses of two thermophilic enzymes exhibiting both beta-1,4 mannosidic and beta-1,4 glucosidic cleavage activities from Caldanaerobius polysaccharolyticus.J Bacteriol. 2010 Aug;192(16):4111-21. doi: 10.1128/JB.00257-10. Epub 2010 Jun 18. J Bacteriol. 2010. PMID: 20562312 Free PMC article.
-
Degradation of microcrystalline cellulose and non-pretreated plant biomass by a cell-free extracellular cellulase/hemicellulase system from the extreme thermophilic bacterium Caldicellulosiruptor bescii.J Biosci Bioeng. 2013 Jan;115(1):64-70. doi: 10.1016/j.jbiosc.2012.07.019. Epub 2012 Aug 23. J Biosci Bioeng. 2013. PMID: 22921519
Cited by
-
Multifunctional alkalophilic α-amylase with diverse raw seaweed degrading activities.AMB Express. 2021 Oct 20;11(1):139. doi: 10.1186/s13568-021-01300-x. AMB Express. 2021. PMID: 34669086 Free PMC article.
-
Insights into Thermophilic Plant Biomass Hydrolysis from Caldicellulosiruptor Systems Biology.Microorganisms. 2020 Mar 10;8(3):385. doi: 10.3390/microorganisms8030385. Microorganisms. 2020. PMID: 32164310 Free PMC article. Review.
-
Extracellular secretion of noncatalytic plant cell wall-binding proteins by the cellulolytic thermophile Caldicellulosiruptor bescii.J Bacteriol. 2014 Nov;196(21):3784-92. doi: 10.1128/JB.01897-14. Epub 2014 Aug 25. J Bacteriol. 2014. PMID: 25157080 Free PMC article.
-
The N-Terminal GH10 Domain of a Multimodular Protein from Caldicellulosiruptor bescii Is a Versatile Xylanase/β-Glucanase That Can Degrade Crystalline Cellulose.Appl Environ Microbiol. 2015 Jun;81(11):3823-33. doi: 10.1128/AEM.00432-15. Epub 2015 Mar 27. Appl Environ Microbiol. 2015. PMID: 25819971 Free PMC article.
-
The GH10 and GH48 dual-functional catalytic domains from a multimodular glycoside hydrolase synergize in hydrolyzing both cellulose and xylan.Biotechnol Biofuels. 2019 Dec 3;12:279. doi: 10.1186/s13068-019-1617-2. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 31827607 Free PMC article.
References
-
- Ahsan M, et al. 1997. Purification and characterization of the family J. catalytic domain derived from the Clostridium thermocellum endoglucanase CelJ. Biosci. Biotechnol. Biochem. 61: 427– 431 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

