Migfilin protein promotes migration and invasion in human glioma through epidermal growth factor receptor-mediated phospholipase C-γ and STAT3 protein signaling pathways
- PMID: 22843679
- PMCID: PMC3463316
- DOI: 10.1074/jbc.M112.393900
Migfilin protein promotes migration and invasion in human glioma through epidermal growth factor receptor-mediated phospholipase C-γ and STAT3 protein signaling pathways
Erratum in
- J Biol Chem. 2013 Apr 12;288(15):10948
Abstract
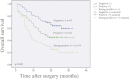
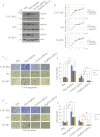
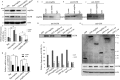
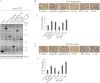
Migfilin is critical for cell shape and motile regulation. However, its pathological role in glioma is unknown. Using an immunohistochemical staining assay, we demonstrate that there is a significant correlation between expression of Migfilin and pathological tumor grade in 217 clinical glioma samples. High Migfilin expression is associated with poor prognosis for patients with glioma. Investigation of the molecular mechanism shows that Migfilin promotes migration and invasion in glioma cells. Moreover, Migfilin positively modulates the expression and activity of epidermal growth factor receptor, and Migfilin-mediated migration and invasion depend on epidermal growth factor receptor-induced PLC-γ and STAT3-signaling pathways. Our results may provide significant clinical application, including use of Migfilin as a molecular marker in glioma for early diagnosis and as an indicator of prognosis.
Figures


References
-
- Furnari F. B., Fenton T., Bachoo R. M., Mukasa A., Stommel J. M., Stegh A., Hahn W. C., Ligon K. L., Louis D. N., Brennan C., Chin L., DePinho R. A., Cavenee W. K. (2007) Malignant astrocytic glioma. Genetics, biology, and paths to treatment. Genes Dev. 21, 2683–2710 - PubMed
-
- Yang L., Ng K. Y., Lillehei K. O. (2003) Cell-mediated immunotherapy. A new approach to the treatment of malignant glioma. Cancer Control 10, 138–147 - PubMed
-
- Stewart L. A. (2002) Chemotherapy in adult high grade glioma. A systematic review and meta-analysis of individual patient data from 12 randomized trials. Lancet 359, 1011–1018 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

