Relative movements of transmembrane regions at the outer mouth of the cystic fibrosis transmembrane conductance regulator channel pore during channel gating
- PMID: 22843683
- PMCID: PMC3442544
- DOI: 10.1074/jbc.M112.385096
Relative movements of transmembrane regions at the outer mouth of the cystic fibrosis transmembrane conductance regulator channel pore during channel gating
Abstract
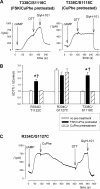
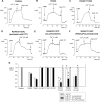
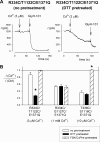
Multiple transmembrane (TM) segments line the pore of the cystic fibrosis transmembrane conductance regulator Cl(-) channel; however, the relative alignment of these TMs and their relative movements during channel gating are unknown. To gain three-dimensional structural information on the outer pore, we have used patch clamp recording to study the proximity of pairs of cysteine side chains introduced into TMs 6 and 11, using both disulfide cross-linking and Cd(2+) coordination. Following channel activation, disulfide bonds could apparently be formed between three cysteine pairs (of 15 studied): R334C/T1122C, R334C/G1127C, and T338C/S1118C. To examine the state dependence of cross-linking, we combined these cysteine mutations with a nucleotide-binding domain mutation (E1371Q) that stabilizes the channel open state. Investigation of the effects of the E1371Q mutation on disulfide bond formation and Cd(2+) coordination suggests that although R334C/T1122C and T338C/S1118C are closer together in the channel open state, R334C/G1127C are close together and can form disulfide bonds only when the channel is closed. These results provide important new information on the three-dimensional structure of the outer mouth of the cystic fibrosis transmembrane conductance regulator channel pore: TMs 6 and 11 are close enough together to form disulfide bonds in both open and closed channels. Moreover, the altered relative locations of residues in open and in closed channels that we infer allow us to propose that channel opening and closing may be associated with a relative translational movement of TMs 6 and 11, with TM6 moving "down" (toward the cytoplasm) during channel opening.
Figures




References
-
- O'Sullivan B. P., Freedman S. D. (2009) Cystic fibrosis. Lancet 373, 1891–1904 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

