The arginine of the DRY motif in transmembrane segment III functions as a balancing micro-switch in the activation of the β2-adrenergic receptor
- PMID: 22843684
- PMCID: PMC3442529
- DOI: 10.1074/jbc.M112.348565
The arginine of the DRY motif in transmembrane segment III functions as a balancing micro-switch in the activation of the β2-adrenergic receptor
Abstract
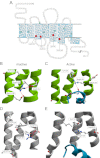
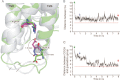
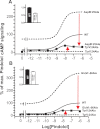
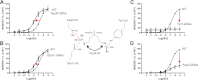
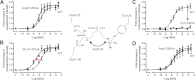
Recent high resolution x-ray structures of the β2-adrenergic receptor confirmed a close salt-bridge interaction between the suspected micro-switch residue ArgIII:26 (Arg3.50) and the neighboring AspIII:25 (Asp3.49). However, neither the expected "ionic lock" interactions between ArgIII:26 and GluVI:-06 (Glu6.30) in the inactive conformation nor the interaction with TyrV:24 (Tyr5.58) in the active conformation were observed in the x-ray structures. Here we find through molecular dynamics simulations, after removal of the stabilizing T4 lysozyme, that the expected salt bridge between ArgIII:26 and GluVI:-06 does form relatively easily in the inactive receptor conformation. Moreover, mutational analysis of GluVI:-06 in TM-VI and the neighboring AspIII:25 in TM-III demonstrated that these two residues do function as locks for the inactive receptor conformation as we observed increased G(s) signaling, arrestin mobilization, and internalization upon alanine substitutions. Conversely, TyrV:24 appears to play a role in stabilizing the active receptor conformation as loss of function of G(s) signaling, arrestin mobilization, and receptor internalization was observed upon alanine substitution of TyrV:24. The loss of function of the TyrV:24 mutant could partly be rescued by alanine substitution of either AspIII:25 or GluVI:-06 in the double mutants. Surprisingly, removal of the side chain of the ArgIII:26 micro-switch itself had no effect on G(s) signaling and internalization and only reduced arrestin mobilization slightly. It is suggested that ArgIII:26 is equally important for stabilizing the inactive and the active conformation through interaction with key residues in TM-III, -V, and -VI, but that the ArgIII:26 micro-switch residue itself apparently is not essential for the actual G protein activation.
Figures
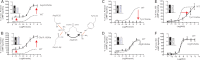
References
-
- Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 - PubMed
-
- Rasmussen S. G., Choi H. J., Rosenbaum D. M., Kobilka T. S., Thian F. S., Edwards P. C., Burghammer M., Ratnala V. R., Sanishvili R., Fischetti R. F., Schertler G. F., Weis W. I., Kobilka B. K. (2007) Crystal structure of the human β2 adrenergic G protein-coupled receptor. Nature 450, 383–387 - PubMed
-
- Rasmussen S. G., Choi H. J., Fung J. J., Pardon E., Casarosa P., Chae P. S., Devree B. T., Rosenbaum D. M., Thian F. S., Kobilka T. S., Schnapp A., Konetzki I., Sunahara R. K., Gellman S. H., Pautsch A., Steyaert J., Weis W. I., Kobilka B. K. (2011) Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180 - PMC - PubMed
-
- Rasmussen S. G., DeVree B. T., Zou Y., Kruse A. C., Chung K. Y., Kobilka T. S., Thian F. S., Chae P. S., Pardon E., Calinski D., Mathiesen J. M., Shah S. T., Lyons J. A., Caffrey M., Gellman S. H., Steyaert J., Skiniotis G., Weis W. I., Sunahara R. K., Kobilka B. K. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

