Novel one-step immunoassays to quantify α-synuclein: applications for biomarker development and high-throughput screening
- PMID: 22843695
- PMCID: PMC3460466
- DOI: 10.1074/jbc.M112.379792
Novel one-step immunoassays to quantify α-synuclein: applications for biomarker development and high-throughput screening
Abstract
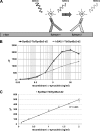
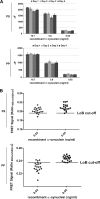
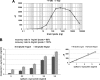
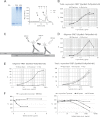
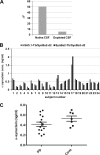
Familial Parkinson disease (PD) can result from α-synuclein gene multiplication, implicating the reduction of neuronal α-synuclein as a therapeutic target. Moreover, α-synuclein content in human cerebrospinal fluid (CSF) represents a PD biomarker candidate. However, capture-based assays for α-synuclein quantification in CSF (such as by ELISA) have shown discrepancies and have limited suitability for high-throughput screening. Here, we describe two sensitive, in-solution, time-resolved Förster's resonance energy transfer (TR-FRET)-based immunoassays for total and oligomeric α-synuclein quantification. CSF analysis showed strong concordance for total α-synuclein content between two TR-FRET assays and, in agreement with a previously characterized 36 h protocol-based ELISA, demonstrated lower α-synuclein levels in PD donors. Critically, the assay suitability for high-throughput screening of siRNA constructs and small molecules aimed at reducing endogenous α-synuclein levels was established and validated. In a small-scale proof of concept compound screen using 384 well plates, signals ranged from <30 to >120% of the mean of vehicle-treated cells for molecules known to lower and increase cellular α-synuclein, respectively. Furthermore, a reverse genetic screen of a kinase-directed siRNA library identified seven genes that modulated α-synuclein protein levels (five whose knockdown increased and two that decreased cellular α-synuclein protein). This provides critical new biological insight into cellular pathways regulating α-synuclein steady-state expression that may help guide further drug discovery efforts. Moreover, we describe an inherent limitation in current α-synuclein oligomer detection methodology, a finding that will direct improvement of future assay design. Our one-step TR-FRET-based platform for α-synuclein quantification provides a novel platform with superior performance parameters for the rapid screening of large biomarker cohorts and of compound and genetic libraries, both of which are essential to the development of PD therapies.
Figures


Similar articles
-
Reduced α-synuclein levels in cerebrospinal fluid in Parkinson's disease are unrelated to clinical and imaging measures of disease severity.Eur J Neurol. 2014 Mar;21(3):388-94. doi: 10.1111/ene.12176. Epub 2013 Apr 30. Eur J Neurol. 2014. PMID: 23631635
-
Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson's and Alzheimer's diseases.Eur J Neurosci. 2016 Jan;43(1):3-16. doi: 10.1111/ejn.13056. Epub 2015 Oct 15. Eur J Neurosci. 2016. PMID: 26332448 Free PMC article.
-
A candidate reference measurement procedure for the quantification of α-synuclein in cerebrospinal fluid using an SI traceable primary calibrator and multiple reaction monitoring.Analyst. 2024 Sep 23;149(19):4842-4850. doi: 10.1039/d4an00634h. Analyst. 2024. PMID: 39041602
-
Value of cerebrospinal fluid α-synuclein species as biomarker in Parkinson's diagnosis and prognosis.Biomark Med. 2016;10(1):35-49. doi: 10.2217/bmm.15.107. Epub 2015 Dec 8. Biomark Med. 2016. PMID: 26643452 Review.
-
Controlling the mass action of alpha-synuclein in Parkinson's disease.J Neurochem. 2008 Oct;107(2):303-16. doi: 10.1111/j.1471-4159.2008.05612.x. Epub 2008 Sep 2. J Neurochem. 2008. PMID: 18691382 Review.
Cited by
-
Assays and technologies for developing proteolysis targeting chimera degraders.Future Med Chem. 2020 Jun;12(12):1155-1179. doi: 10.4155/fmc-2020-0073. Epub 2020 May 20. Future Med Chem. 2020. PMID: 32431173 Free PMC article. Review.
-
Modelling idiopathic Parkinson disease as a complex illness can inform incidence rate in healthy adults: the PR EDIGT score.Eur J Neurosci. 2017 Jan;45(1):175-191. doi: 10.1111/ejn.13476. Epub 2016 Dec 27. Eur J Neurosci. 2017. PMID: 27859866 Free PMC article.
-
Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of "Protein-Misfolding Cyclic Amplification" and "Real-Time Quaking-Induced Conversion" as Diagnostic Tools.Front Neurol. 2018 Jun 6;9:415. doi: 10.3389/fneur.2018.00415. eCollection 2018. Front Neurol. 2018. PMID: 29928254 Free PMC article. Review.
-
Alpha-synuclein post-translational modifications as potential biomarkers for Parkinson disease and other synucleinopathies.Mol Cell Proteomics. 2013 Dec;12(12):3543-58. doi: 10.1074/mcp.R113.032730. Epub 2013 Aug 21. Mol Cell Proteomics. 2013. PMID: 23966418 Free PMC article. Review.
-
Ultrasensitive Detection of Aggregated α-Synuclein in Glial Cells, Human Cerebrospinal Fluid, and Brain Tissue Using the RT-QuIC Assay: New High-Throughput Neuroimmune Biomarker Assay for Parkinsonian Disorders.J Neuroimmune Pharmacol. 2019 Sep;14(3):423-435. doi: 10.1007/s11481-019-09835-4. Epub 2019 Jan 31. J Neuroimmune Pharmacol. 2019. PMID: 30706414 Free PMC article.
References
-
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 - PubMed
-
- Cooper A. A., Gitler A. D., Cashikar A., Haynes C. M., Hill K. J., Bhullar B., Liu K., Xu K., Strathearn K. E., Liu F., Cao S., Caldwell K. A., Caldwell G. A., Marsischky G., Kolodner R. D., Labaer J., Rochet J. C., Bonini N. M., Lindquist S. (2006) α-Synuclein blocks ER-Golgi traffic, and Rab1 rescues neuron loss in Parkinson models. Science 313, 324–328 - PMC - PubMed
-
- Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. (2003) α-Synuclein locus triplication causes Parkinson disease. Science 302, 841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

