Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains
- PMID: 22843783
- PMCID: PMC3657521
- DOI: 10.1093/cid/cis574
Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains
Abstract
Background: Influenza vaccines may be reformulated annually because of antigenic drift in influenza viruses. However, the relationship between antigenic characteristics of circulating viruses and vaccine effectiveness (VE) is not well understood. We conducted an assessment of the effectiveness of US influenza vaccines during the 2010-2011 season.
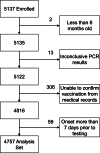
Methods: We performed a case-control study comparing vaccination histories between subjects with acute respiratory illness with positive real-time reverse transcription polymerase chain reaction for influenza and influenza test-negative controls. Subjects with acute respiratory illness of ≤7 days duration were enrolled in hospitals, emergency departments, or outpatient clinics in communities in 4 states. History of immunization with the 2010-2011 vaccine was ascertained from vaccine registries or medical records. Vaccine effectiveness was estimated in logistic regression models adjusted for study community, age, race, insurance status, enrollment site, and presence of a high-risk medical condition.
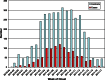
Results: A total of 1040 influenza-positive cases and 3717 influenza-negative controls were included from the influenza season, including 373 cases of influenza A(H1N1), 334 cases of influenza A(H3N2), and 333 cases of influenza B. Overall adjusted VE was 60% (95% confidence interval [CI], 53%-66%). Age-specific VE estimates ranged from 69% (95% CI, 56%-77%) in children aged 6 months-8 years to 38% (95% CI, -16% to 67%) in adults aged ≥65 years.
Conclusions: The US 2010-2011 influenza vaccines were moderately effective in preventing medically attended influenza during a season when all 3 vaccine strains were antigenically similar to circulating viruses. Continued monitoring of influenza vaccines in all age groups is important, particularly as new vaccines are introduced.
Figures
References
-
- Orenstein EW, de Serres G, Haber MJ, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–31. - PubMed
-
- Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1–62.
-
- Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influena vaccination status in a Wisconsin cohort. Vaccine. 2009;27:6543–9. - PubMed
-
- Kendal A, Pereira M, Skehel J. Hemagglutination-inhibtion: concepts and procedures for laboratory-based influenza surveillance. 1982 US Department of Health and Human Services, Atlanta GA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

