Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency
- PMID: 22843797
- PMCID: PMC3492833
- DOI: 10.1152/ajpcell.00191.2012
Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency
Abstract
Clinical trials of bone marrow mesenchymal stem cell (MSC) therapy have thus far demonstrated moderate and inconsistent benefits, indicating an urgent need to improve therapeutic efficacy. Although administration of sufficient cells is necessary to achieve maximal therapeutic benefits, documented MSC clinical trials have largely relied on injections of ∼1 × 10(6) cells/kg, which appears too low to elicit a robust therapeutic response according to published preclinical studies. However, repeated cell passaging necessary for large-scale expansion of MSC causes cellular senescence and reduces stem cell potency. Using the RNA mimetic polyinosinic-polycytidylic acid [poly(I:C)] to engage MSC Toll-like receptor 3 (TLR3), we found that poly(I:C), signaling through multiple mitogen-activated protein kinase pathways, induced therapeutically relevant trophic factors such as interleukin-6-type cytokines, stromal-derived factor 1, hepatocyte growth factor, and vascular endothelial growth factor while slightly inhibiting the proliferation and migration potentials of MSC. At the suboptimal injection dose of 1 × 10(6) cells/kg, poly(I:C)-treated MSC, but not untreated MSC, effectively stimulated regeneration of the failing hamster heart 1 mo after cell administration. The regenerating heart exhibited increased CD34(+)/Ki67(+) and CD34(+)/GATA4(+) progenitor cells in the presence of decreased inflammatory cells and cytokines. Cardiac functional improvement was associated with a ∼50% reduction in fibrosis, a ∼40% reduction in apoptosis, and a ∼55% increase in angiogenesis, culminating in prominent cardiomyogenesis evidenced by abundant distribution of small myocytes and a ∼90% increase in wall thickening. These functional, histological, and molecular characterizations thus establish the utility of TLR3 engagement for enabling the low-dose MSC therapy that may be translated to more efficacious clinical applications.
Figures

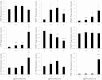



 P < 0.001 vs. 1 × 106 MSC/kg;
P < 0.001 vs. 1 × 106 MSC/kg;  P < 0.001 vs. 1 × 106 MSC-IC/kg.
P < 0.001 vs. 1 × 106 MSC-IC/kg.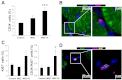




Comment in
-
Mesenchymal stem cells and cardiac regeneration: a sophisticated approach depends on trophic effects--what's left over? Focus on "Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency".Am J Physiol Cell Physiol. 2012 Nov 15;303(10):C1004-5. doi: 10.1152/ajpcell.00295.2012. Epub 2012 Sep 12. Am J Physiol Cell Physiol. 2012. PMID: 22972800 No abstract available.
References
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732– 738, 2001 - PubMed
-
- Allison M. Genzyme backs Osiris, despite Prochymal flop. Nat Biotechnol 27: 966– 967, 2009 - PubMed
-
- Alreja G, Bugano D, Lotfi A. Effect of remote ischemic preconditioning on myocardial and renal injury: meta-analysis of randomized controlled trials. J Invasive Cardiol 24: 42– 48, 2012 - PubMed
-
- Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108: 863– 868, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

