Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus
- PMID: 22843840
- PMCID: PMC3457211
- DOI: 10.1128/JB.00743-12
Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus
Abstract
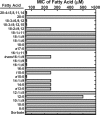
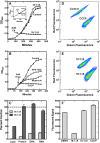
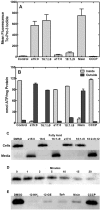
The skin represents an important barrier for pathogens and is known to produce fatty acids that are toxic toward gram-positive bacteria. A screen of fatty acids as growth inhibitors of Staphylococcus aureus revealed structure-specific antibacterial activity. Fatty acids like oleate (18:1Δ9) were nontoxic, whereas palmitoleate (16:1Δ9) was a potent growth inhibitor. Cells treated with 16:1Δ9 exhibited rapid membrane depolarization, the disruption of all major branches of macromolecular synthesis, and the release of solutes and low-molecular-weight proteins into the medium. Other cytotoxic lipids, such as glycerol ethers, sphingosine, and acyl-amines blocked growth by the same mechanisms. Nontoxic 18:1Δ9 was used for phospholipid synthesis, whereas toxic 16:1Δ9 was not and required elongation to 18:1Δ11 prior to incorporation. However, blocking fatty acid metabolism using inhibitors to prevent acyl-acyl carrier protein formation or glycerol-phosphate acyltransferase activity did not increase the toxicity of 18:1Δ9, indicating that inefficient metabolism did not play a determinant role in fatty acid toxicity. Nontoxic 18:1Δ9 was as toxic as 16:1Δ9 in a strain lacking wall teichoic acids and led to growth arrest and enhanced release of intracellular contents. Thus, wall teichoic acids contribute to the structure-specific antimicrobial effects of unsaturated fatty acids. The ability of poorly metabolized 16:1 isomers to penetrate the cell wall defenses is a weakness that has been exploited by the innate immune system to combat S. aureus.
Figures


References
-
- Balemans W, et al. 2010. Essentiality of FASII pathway for Staphylococcus aureus. Nature 463:E3 doi:10.1038/nature08667 - DOI - PubMed
-
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917 - PubMed
-
- Brinster S, et al. 2009. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature 458:83–86 - PubMed
-
- Brinster S, et al. 2010. Reply to “Essentiality of FASII pathway for Staphylococcus aureus.” Nature 463:E4 doi:10.1038/nature08668 - DOI - PubMed
-
- Butcher GW, King G, Dyke KG. 1976. Sensitivity of Staphylococcus aureus to unsaturated fatty acids. J. Gen. Microbiol. 94:290–296 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

