Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses
- PMID: 22844110
- PMCID: PMC3424321
- DOI: 10.4049/jimmunol.1102892
Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses
Abstract
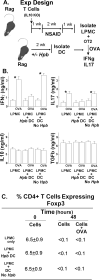
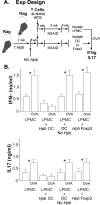
Immunological diseases such as inflammatory bowel disease (IBD) are infrequent in less developed countries, possibly because helminths provide protection by modulating host immunity. In IBD murine models, the helminth Heligmosomoides polygyrus bakeri prevents colitis. It was determined whether H. polygyrus bakeri mediated IBD protection by altering dendritic cell (DC) function. We used a Rag IBD model where animals were reconstituted with IL10⁻/⁻ T cells, making them susceptible to IBD and with OVA Ag-responsive OT2 T cells, allowing study of a gut antigenic response. Intestinal DC from H. polygyrus bakeri-infected Rag mice added to lamina propria mononuclear cells (LPMC) isolated from colitic animals blocked OVA IFN-γ/IL-17 responses in vitro through direct contact with the inflammatory LPMC. DC from uninfected Rag mice displayed no regulatory activity. Transfer of DC from H. polygyrus bakeri-infected mice into Rag mice reconstituted with IL10⁻/⁻ T cells protected animals from IBD, and LPMC from these mice lost OVA responsiveness. After DC transfer, OT2 T cells populated the intestines normally. However, the OT2 T cells were rendered Ag nonresponsive through regulatory action of LPMC non-T cells. The process of regulation appeared to be regulatory T cell independent. Thus, H. polygyrus bakeri modulates intestinal DC function, rendering them tolerogenic. This appears to be an important mechanism through which H. polygyrus bakeri suppresses colitis. IFN-γ and IL-17 are colitogenic. The capacity of these DC to block a gut Ag-specific IFN-γ/IL-17 T cell response also is significant.
Figures
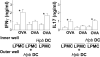



Similar articles
-
Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity.J Immunol. 2010 Sep 15;185(6):3184-9. doi: 10.4049/jimmunol.1000941. Epub 2010 Aug 11. J Immunol. 2010. PMID: 20702728 Free PMC article.
-
Heligmosomoides polygyrus bakeri infection activates colonic Foxp3+ T cells enhancing their capacity to prevent colitis.J Immunol. 2013 Aug 15;191(4):1927-34. doi: 10.4049/jimmunol.1201457. Epub 2013 Jul 12. J Immunol. 2013. PMID: 23851695 Free PMC article.
-
Heligmosomoides polygyrus abrogates antigen-specific gut injury in a murine model of inflammatory bowel disease.Inflamm Bowel Dis. 2012 Aug;18(8):1447-55. doi: 10.1002/ibd.22858. Epub 2012 Jan 4. Inflamm Bowel Dis. 2012. PMID: 22223533 Free PMC article.
-
Immune modulation and modulators in Heligmosomoides polygyrus infection.Exp Parasitol. 2012 Sep;132(1):76-89. doi: 10.1016/j.exppara.2011.08.011. Epub 2011 Aug 22. Exp Parasitol. 2012. PMID: 21875581 Free PMC article. Review.
-
Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease.World J Gastroenterol. 2011 Sep 7;17(33):3761-75. doi: 10.3748/wjg.v17.i33.3761. World J Gastroenterol. 2011. PMID: 21987618 Free PMC article. Review.
Cited by
-
Production and analysis of immunomodulatory excretory-secretory products from the mouse gastrointestinal nematode Heligmosomoides polygyrus bakeri.Nat Protoc. 2014 Dec;9(12):2740-54. doi: 10.1038/nprot.2014.184. Epub 2014 Nov 6. Nat Protoc. 2014. PMID: 25375989
-
Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications.BMC Med. 2015 Apr 13;13:81. doi: 10.1186/s12916-015-0306-7. BMC Med. 2015. PMID: 25879741 Free PMC article. Review.
-
A clinical review of recent findings in the epidemiology of inflammatory bowel disease.Clin Epidemiol. 2013 Jul 25;5:237-47. doi: 10.2147/CLEP.S33961. Print 2013. Clin Epidemiol. 2013. PMID: 23922506 Free PMC article.
-
Worms: Pernicious parasites or allies against allergies?Parasite Immunol. 2019 Jun;41(6):e12574. doi: 10.1111/pim.12574. Epub 2018 Aug 29. Parasite Immunol. 2019. PMID: 30043455 Free PMC article. Review.
-
Helminth Lessons in Inflammatory Bowel Diseases (IBD).Biomedicines. 2023 Apr 18;11(4):1200. doi: 10.3390/biomedicines11041200. Biomedicines. 2023. PMID: 37189818 Free PMC article. Review.
References
-
- Elliott DE, Urban JF, Jr., Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn's disease? FASEB J. 2000;14:1848–1855. - PubMed
-
- Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr., Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

