Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage
- PMID: 22844119
- PMCID: PMC3424306
- DOI: 10.4049/jimmunol.1103545
Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage
Abstract
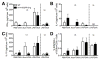
Lymphatic vessels transport interstitial fluid, soluble Ag, and immune cells from peripheral tissues to lymph nodes (LNs), yet the contribution of peripheral lymphatic drainage to adaptive immunity remains poorly understood. We examined immune responses to dermal vaccination and contact hypersensitivity (CHS) challenge in K14-VEGFR-3-Ig mice, which lack dermal lymphatic capillaries and experience markedly depressed transport of solutes and dendritic cells from the skin to draining LNs. In response to dermal immunization, K14-VEGFR-3-Ig mice produced lower Ab titers. In contrast, although delayed, T cell responses were robust after 21 d, including high levels of Ag-specific CD8+ T cells and production of IFN-γ, IL-4, and IL-10 upon restimulation. T cell-mediated CHS responses were strong in K14-VEGFR-3-Ig mice, but importantly, their ability to induce CHS tolerance in the skin was impaired. In addition, 1-y-old mice displayed multiple signs of autoimmunity. These data suggest that lymphatic drainage plays more important roles in regulating humoral immunity and peripheral tolerance than in effector T cell immunity.
Figures


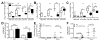



References
-
- Friedlaender MH, Baer H. Immunologic tolerance: role of the regional lymph node. Science. 1972;176:312–314. - PubMed
-
- Friedlaender MH, Chisari FV, Baer H. The role of the inflammatory response of skin and lymph nodes in the induction of sensitization to simple chemicals. J Immunol. 1973;111:164–170. - PubMed
-
- Hintzen G, Ohl L, del Rio ML, Rodriguez-Barbosa JI, Pabst O, Kocks JR, Krege J, Hardtke S, Forster R. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol. 2006;177:7346–7354. - PubMed
-
- Reynoso ED, Lee JW, Turley SJ. Peripheral tolerance induction by lymph node stroma. In: Schoenberger SP, Katsikis PD, Pulendran B, editors. Crossroads between Innate and Adaptive Immunity Ii. 2009. pp. 113–127.
-
- Baird JB, Charles JL, Streit TG, Roberts JM, Addiss DG, Lammie PJ. Reactivity to bacterial, fungal, and parasite antigens in patients with lymphedema and elephantiasis. Am J Trop Med Hyg. 2002;66:163–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

