Drosophila fatty acid transport protein regulates rhodopsin-1 metabolism and is required for photoreceptor neuron survival
- PMID: 22844251
- PMCID: PMC3405995
- DOI: 10.1371/journal.pgen.1002833
Drosophila fatty acid transport protein regulates rhodopsin-1 metabolism and is required for photoreceptor neuron survival
Abstract
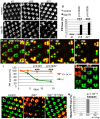
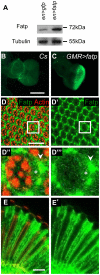
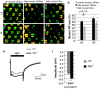
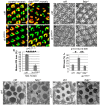
Tight regulation of the visual response is essential for photoreceptor function and survival. Visual response dysregulation often leads to photoreceptor cell degeneration, but the causes of such cell death are not well understood. In this study, we investigated a fatty acid transport protein (fatp) null mutation that caused adult-onset and progressive photoreceptor cell death. Consistent with fatp having a role in the retina, we showed that fatp is expressed in adult photoreceptors and accessory cells and that its re-expression in photoreceptors rescued photoreceptor viability in fatp mutants. The visual response in young fatp-mutant flies was abnormal with elevated electroretinogram amplitudes associated with high levels of Rhodopsin-1 (Rh1). Reducing Rh1 levels in rh1 mutants or depriving flies of vitamin A rescued photoreceptor cell death in fatp mutant flies. Our results indicate that fatp promotes photoreceptor survival by regulating Rh1 abundance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Drosophila fabp is required for light-dependent Rhodopsin-1 clearance and photoreceptor survival.PLoS Genet. 2021 Oct 29;17(10):e1009551. doi: 10.1371/journal.pgen.1009551. eCollection 2021 Oct. PLoS Genet. 2021. PMID: 34714826 Free PMC article.
-
Drosophila king tubby (ktub) mediates light-induced rhodopsin endocytosis and retinal degeneration.J Biomed Sci. 2012 Dec 10;19(1):101. doi: 10.1186/1423-0127-19-101. J Biomed Sci. 2012. PMID: 23228091 Free PMC article.
-
The Gos28 SNARE protein mediates intra-Golgi transport of rhodopsin and is required for photoreceptor survival.J Biol Chem. 2014 Nov 21;289(47):32392-409. doi: 10.1074/jbc.M114.585166. Epub 2014 Sep 26. J Biol Chem. 2014. PMID: 25261468 Free PMC article.
-
Rhodopsin mutations as the cause of retinal degeneration. Classification of degeneration phenotypes in the model system Drosophila melanogaster.Acta Anat (Basel). 1998;162(2-3):85-94. doi: 10.1159/000046472. Acta Anat (Basel). 1998. PMID: 9831754 Review.
-
Molecular genetics of retinal degeneration: A Drosophila perspective.Fly (Austin). 2011 Oct-Dec;5(4):356-68. doi: 10.4161/fly.5.4.17809. Epub 2011 Sep 7. Fly (Austin). 2011. PMID: 21897116 Free PMC article. Review.
Cited by
-
Whole Genome Sequencing of Giant Schnauzer Dogs with Progressive Retinal Atrophy Establishes NECAP1 as a Novel Candidate Gene for Retinal Degeneration.Genes (Basel). 2019 May 21;10(5):385. doi: 10.3390/genes10050385. Genes (Basel). 2019. PMID: 31117272 Free PMC article.
-
Drosophila fabp is required for light-dependent Rhodopsin-1 clearance and photoreceptor survival.PLoS Genet. 2021 Oct 29;17(10):e1009551. doi: 10.1371/journal.pgen.1009551. eCollection 2021 Oct. PLoS Genet. 2021. PMID: 34714826 Free PMC article.
-
Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases.Nat Rev Neurosci. 2014 Apr;15(4):233-49. doi: 10.1038/nrn3689. Epub 2014 Mar 12. Nat Rev Neurosci. 2014. PMID: 24619348 Review.
-
Fatty acid transport protein 1 regulates retinoid metabolism and photoreceptor development in mouse retina.PLoS One. 2017 Jul 3;12(7):e0180148. doi: 10.1371/journal.pone.0180148. eCollection 2017. PLoS One. 2017. PMID: 28672005 Free PMC article.
-
Injection of seminal fluid into the hemocoel of honey bee queens (Apis mellifera) can stimulate post-mating changes.Sci Rep. 2020 Jul 20;10(1):11990. doi: 10.1038/s41598-020-68437-w. Sci Rep. 2020. PMID: 32686702 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

