Interleukin-12p70 expression by dendritic cells of HIV-1-infected patients fails to stimulate gag-specific immune responses
- PMID: 22844321
- PMCID: PMC3401557
- DOI: 10.1155/2012/184979
Interleukin-12p70 expression by dendritic cells of HIV-1-infected patients fails to stimulate gag-specific immune responses
Abstract
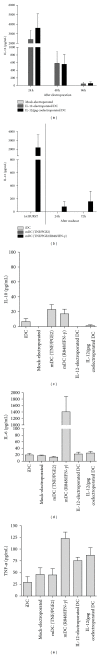
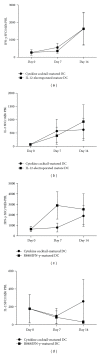
A variety of immune-based therapies has been developed in order to boost or induce protective CD8(+) T cell responses in order to control HIV replication. Since dendritic cells (DCs) are professional antigen-presenting cells (APCs) with the unique capability to stimulate naïve T cells into effector T cells, their use for the induction of HIV-specific immune responses has been studied intensively. In the present study we investigated whether modulation of the activation state of DCs electroporated with consensus codon-optimized HxB2 gag mRNA enhances their capacity to induce HIV gag-specific T cell responses. To this end, mature DCs were (i) co-electroporated with mRNA encoding interleukin (IL)-12p70 mRNA, or (ii) activated with a cytokine cocktail consisting of R848 and interferon (IFN)-γ. Our results confirm the ability of HxB2 gag-expressing DCs to expand functional HIV-specific CD8(+) T cells. However, although most of the patients had detectable gag-specific CD8(+) T cell responses, no significant differences in the level of expansion of functional CD8(+) T cells could be demonstrated when comparing conventional or immune-modulated DCs expressing IL-12p70. This result which goes against expectation may lead to a re-evaluation of the need for IL-12 expression by DCs in order to improve T-cell responses in HIV-1-infected individuals.
Figures

Similar articles
-
Efficient in vitro expansion of human immunodeficiency virus (HIV)-specific T-cell responses by gag mRNA-electroporated dendritic cells from treated and untreated HIV type 1-infected individuals.J Virol. 2008 Apr;82(7):3561-73. doi: 10.1128/JVI.02080-07. Epub 2008 Jan 30. J Virol. 2008. PMID: 18234800 Free PMC article.
-
Efficient stimulation of HIV-1-specific T cells using dendritic cells electroporated with mRNA encoding autologous HIV-1 Gag and Env proteins.Blood. 2006 Mar 1;107(5):1818-27. doi: 10.1182/blood-2005-01-0339. Epub 2005 Nov 1. Blood. 2006. PMID: 16263796
-
mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients.AIDS. 2012 Feb 20;26(4):F1-12. doi: 10.1097/QAD.0b013e32834f33e8. AIDS. 2012. PMID: 22156965 Clinical Trial.
-
Role of Dendritic Cells in Natural Immune Control of HIV-1 Infection.Front Immunol. 2019 Jun 6;10:1306. doi: 10.3389/fimmu.2019.01306. eCollection 2019. Front Immunol. 2019. PMID: 31244850 Free PMC article. Review.
-
TTP-mediated regulation of mRNA stability in immune cells contributes to adaptive immunity, immune tolerance and clinical applications.RNA Biol. 2021 Dec;18(12):2150-2156. doi: 10.1080/15476286.2021.1917185. Epub 2021 Apr 27. RNA Biol. 2021. PMID: 33866923 Free PMC article. Review.
Cited by
-
Differential partial activation phenotype and production of tumour necrosis factor-α by conventional dendritic cells in response to lipopolysaccharide in HIV+ viraemic subjects and HIV+ controllers.Clin Exp Immunol. 2014 Dec;178(3):489-503. doi: 10.1111/cei.12430. Clin Exp Immunol. 2014. PMID: 25130456 Free PMC article.
-
Using Dendritic Cell-Based Immunotherapy to Treat HIV: How Can This Strategy be Improved?Front Immunol. 2018 Dec 18;9:2993. doi: 10.3389/fimmu.2018.02993. eCollection 2018. Front Immunol. 2018. PMID: 30619346 Free PMC article. Review.
References
-
- Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature Immunology. 2002;3(11):1061–1068. - PubMed
-
- Hess C, Altfeld M, Thomas SY, et al. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. The Lancet. 2004;363(9412):863–866. - PubMed
-
- Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nature Medicine. 2004;10(8):806–810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

