Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity
- PMID: 22844426
- PMCID: PMC3406031
- DOI: 10.1371/journal.pone.0041079
Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity
Abstract
Background: Associations have been made between obesity and reduced intestinal numbers of members of the phylum Bacteroidetes, but there is no direct evidence of the role these bacteria play in obesity. Herein, the effects of Bacteroides uniformis CECT 7771 on obesity-related metabolic and immune alterations have been evaluated.
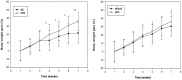
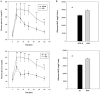
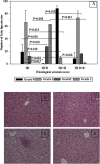
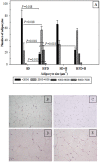
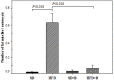
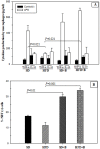
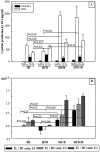
Methods and findings: Adult (6-8 week) male wild-type C57BL-6 mice were fed a standard diet or a high-fat-diet HFD to induce obesity, supplemented or not with B. uniformis CECT 7771 for seven weeks. Animal weight was monitored and histologic, biochemical, immunocompetent cell functions, and features of the faecal microbiota were analysed after intervention. The oral administration of B. uniformis CECT 7771 reduced body weight gain, liver steatosis and liver cholesterol and triglyceride concentrations and increased small adipocyte numbers in HFD-fed mice. The strain also reduced serum cholesterol, triglyceride, glucose, insulin and leptin levels, and improved oral tolerance to glucose in HFD fed mice. The bacterial strain also reduced dietary fat absorption, as indicated by the reduced number of fat micelles detected in enterocytes. Moreover, B. uniformis CECT 7771 improved immune defence mechanisms, impaired in obesity. HFD-induced obesity led to a decrease in TNF-α production by peritoneal macrophages stimulated with LPS, conversely, the administration of B. uniformis CECT 7771 increased TNF-α production and phagocytosis. Administering this strain also increased TNF-α production by dendritic cells (DCs) in response to LPS stimulation, which was significantly reduced by HFD. B. uniformis CECT 7771 also restored the capacity of DCs to induce a T-cell proliferation response, which was impaired in obese mice. HFD induced marked changes in gut microbiota composition, which were partially restored by the intervention.
Conclusions: Altogether, the findings indicate that administration of B. uniformis CECT 7771 ameliorates HFD-induced metabolic and immune dysfunction associated with intestinal dysbiosis in obese mice.
Conflict of interest statement
Figures

References
-
- Moreno LA, Mesana MI, Gonzalez-Gross M, Gil CM, Fleta J, et al. (2006) Anthropometric body fat composition reference values in Spanish adolescents. The AVENA Study. Eur J Clin Nutr 60: 191–196. - PubMed
-
- Badman MK, Flier JS (2007) The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 132: 2103–2115. - PubMed
-
- Sanz Y, Santacruz A, Gauffin P (2010) Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc 69: 434–441. - PubMed
-
- Verwaerde C, Delanoye A, Macia L, Tailleux A, Wolowczuk I (2006) Influence of high-fat feeding on both naive and antigen-experienced T-cell immune response in DO10.11 mice. Scand J Immunol 64: 457–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

