Estrogens induce expression of membrane-associated estrogen receptor α isoforms in lactotropes
- PMID: 22844453
- PMCID: PMC3402499
- DOI: 10.1371/journal.pone.0041299
Estrogens induce expression of membrane-associated estrogen receptor α isoforms in lactotropes
Abstract
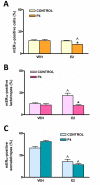
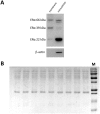
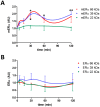
Estrogens are key to anterior pituitary function, stimulating hormone release and controlling cell fate to achieve pituitary dynamic adaptation to changing physiological conditions. In addition to their classical mechanism of action through intracellular estrogen receptors (ERs), estrogens exert rapid actions via cell membrane-localized ERs (mERs). We previously showed that E2 exerts a rapid pro-apoptotic action in anterior pituitary cells, especially in lactotropes and somatotropes, through activation of mERs. In the present study, we examined the involvement of mERα in the rapid pro-apoptotic action of estradiol by TUNEL in primary cultures of anterior pituitary cells from ovariectomized rats using a cell-impermeable E2 conjugate (E2-BSA) and an ERα selective antagonist (MPP dihydrochloride). We studied mERα expression during the estrous cycle and its regulation by gonadal steroids in vivo by flow cytometry. We identified ERα variants in the plasma membrane of anterior pituitary cells during the estrous cycle and studied E2 regulation of these mERα variants in vitro by surface biotinylation and Western Blot. E2-BSA-induced apoptosis was abrogated by MPP in total anterior pituitary cells and lactotropes. In cycling rats, we detected a higher number of lactotropes and a lower number of somatotropes expressing mERα at proestrus than at diestrus. Acute E2 treatment increased the percentage of mERα-expressing lactotropes whereas it decreased the percentage of mERα-expressing somatotropes. We detected three mERα isoforms of 66, 39 and 22 kDa. Expression of mERα66 and mERα39 was higher at proestrus than at diestrus, and short-term E2 incubation increased expression of these two mERα variants. Our results indicate that the rapid apoptotic action exerted by E2 in lactotropes depends on mERα, probably full-length ERα and/or a 39 kDa ERα variant. Expression and activation of mERα variants in lactotropes could be one of the mechanisms through which E2 participates in anterior pituitary cell renewal during the estrous cycle.
Conflict of interest statement
Figures
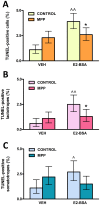
 <0.01 vs respective control without E2-BSA, χ2 test.
<0.01 vs respective control without E2-BSA, χ2 test.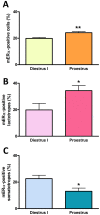

References
-
- Shupnik MA. Oestrogen receptors, receptor variants and oestrogen actions in the hypothalamic-pituitary axis. J Neuroendocrinol. 2002;14:85–94. - PubMed
-
- Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Rec Prog Horm Res. 1962;18:387–414.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

