Thyroid hormone enhances nitric oxide-mediated bacterial clearance and promotes survival after meningococcal infection
- PMID: 22844479
- PMCID: PMC3402396
- DOI: 10.1371/journal.pone.0041445
Thyroid hormone enhances nitric oxide-mediated bacterial clearance and promotes survival after meningococcal infection
Abstract
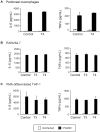
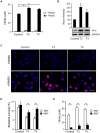
Euthyroid sick syndrome characterized by reduced levels of thyroid hormones (THs) is observed in patients with meningococcal shock. It has been found that the level of THs reflects disease severity and is predictive for mortality. The present study was conducted to investigate the impact of THs on host defense during meningococcal infection. We found that supplementation of thyroxine to mice infected with Neisseria meningitidis enhanced bacterial clearance, attenuated the inflammatory responses and promoted survival. In vitro studies with macrophages revealed that THs enhanced bacteria-cell interaction and intracellular killing of meningococci by stimulating inducible nitric oxide synthase (iNos)-mediated NO production. TH treatment did not activate expression of TH receptors in macrophages. Instead, the observed TH-directed actions were mediated through nongenomic pathways involving the protein kinases PI3K and ERK1/2 and initiated at the membrane receptor integrin αvβ3. Inhibition of nongenomic TH signaling prevented iNos induction, NO production and subsequent intracellular bacterial killing by macrophages. These data demonstrate a beneficial role of THs in macrophage-mediated N. meningitidis clearance. TH replacement might be a novel option to control meningococcal septicemia.
Conflict of interest statement
Figures






References
-
- Kelly GS. Peripheral metabolism of thyroid hormones: a review. Altern Med Rev. 2000;5:306–333. - PubMed
-
- Flamant F, Gauthier K, Samarut J. Thyroid hormones signaling is getting more complex: STORMs are coming. Mol Endocrinol. 2007;21:321–333. - PubMed
-
- Bassett JH, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol. 2003;213:1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

