Protein tyrosine phosphatase PTP1B is involved in hippocampal synapse formation and learning
- PMID: 22844492
- PMCID: PMC3402386
- DOI: 10.1371/journal.pone.0041536
Protein tyrosine phosphatase PTP1B is involved in hippocampal synapse formation and learning
Abstract
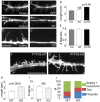
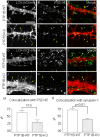
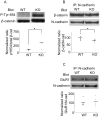
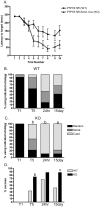
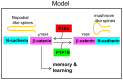
ER-bound PTP1B is expressed in hippocampal neurons, and accumulates among neurite contacts. PTP1B dephosphorylates ß-catenin in N-cadherin complexes ensuring cell-cell adhesion. Here we show that endogenous PTP1B, as well as expressed GFP-PTP1B, are present in dendritic spines of hippocampal neurons in culture. GFP-PTP1B overexpression does not affect filopodial density or length. In contrast, impairment of PTP1B function or genetic PTP1B-deficiency leads to increased filopodia-like dendritic spines and a reduction in mushroom-like spines, while spine density is unaffected. These morphological alterations are accompanied by a disorganization of pre- and post-synapses, as judged by decreased clustering of synapsin-1 and PSD-95, and suggest a dynamic synaptic phenotype. Notably, levels of ß-catenin-Tyr-654 phosphorylation increased ∼5-fold in the hippocampus of adult PTP1B(-/-) (KO) mice compared to wild type (WT) mice and this was accompanied by a reduction in the amount of ß-catenin associated with N-cadherin. To determine whether PTP1B-deficiency alters learning and memory, we generated mice lacking PTP1B in the hippocampus and cortex (PTP1B(fl/fl)-Emx1-Cre). PTP1B(fl/fl)-Emx1-Cre mice displayed improved performance in the Barnes maze (decreased time to find and enter target hole), utilized a more efficient strategy (cued), and had better recall compared to WT controls. Our results implicate PTP1B in structural plasticity within the hippocampus, likely through modulation of N-cadherin function by ensuring dephosphorylation of ß-catenin on Tyr-654. Disruption of hippocampal PTP1B function or expression leads to elongation of dendritic filopodia and improved learning and memory, demonstrating an exciting novel role for this phosphatase.
Conflict of interest statement
Figures


References
-
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. - PubMed
-
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. - PubMed
-
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

