Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome
- PMID: 22844557
- PMCID: PMC3404166
- DOI: 10.5009/gnl.2012.6.3.305
Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome
Abstract
The etiopathogenesis of the irritable bowel syndrome (IBS), one of the most prevalent gastrointestinal disorders, is not well known. The most accepted hypothesis is that IBS is the result of the disturbance of the 'brain-gut axis.' Although the pathophysiological mechanisms of intestinal dysfunction are complex and not completely understood, stress, infections, gut flora, and altered immune response are thought to play a role in IBS development. The intestinal barrier, composed of a single-cell layer, forms a physical barrier that separates the intestinal lumen from the internal milieu. The loss of integrity of this barrier is related with mucosal immune activation and intestinal dysfunction in IBS. The number of mast cells and T lymphocytes is increased in the intestinal mucosa of certain IBS patients, and the mediators released by these cells could compromise the epithelial barrier function and alter nerve signaling within the enteric nervous system. The association of clinical symptoms to structural and functional abnormalities of the mucosal barrier in IBS patients highlights the importance of understanding the physiological role of the gut barrier in the pathogenesis of this disorder. This review summarizes the clinical and experimental evidences indicating the cellular and molecular mechanisms of IBS symptomatology, and its relevance for future translational research.
Keywords: Intestinal barrier function; Irritable bowel syndrome; Mast cells; Tight junctions.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
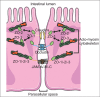
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

