Concise Total Synthesis of (+)-Gliocladins B and C
- PMID: 22844577
- PMCID: PMC3404843
- DOI: 10.1039/C2SC20270K
Concise Total Synthesis of (+)-Gliocladins B and C
Abstract
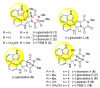
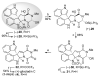
The first total synthesis of (+)-gliocladin B is described. Our concise and enantioselective synthesis takes advantage of a new regioselective Friedel-Crafts-based strategy to provide an efficient multigram-scale access to the C3-(3'-indolyl)hexahydropyrroloindole substructure, a molecular foundation present in a significant subset of epipolythiodiketopiperazine natural alkaloids. Our first-generation solution to (+)-gliocladin B involved the stereoselective formation of (+)-12-deoxybionectin A, a plausible biosynthetic precursor. Our synthesis clarified the C15 stereochemistry of (+)-gliocladin B and allowed its full structure confirmation. Further studies of a versatile dihydroxylated diketopiperazine provided a concise and efficient synthesis of (+)-gliocladin B as well as access to (+)-gliocladin C.
Figures


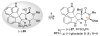
References
-
-
For reviews on cyclotryptophan and cyclotryptamine alkaloids, see: Anthoni U, Christophersen C, Nielsen PH. ch. 2. In: Pelletier SW, editor. Alkaloids: Chemical and Biological Perspectives. Vol. 13. Pergamon Press; London: 1999. pp. 163–236.; Hino T, Nakagawa M. ch. 1. In: Brossi A, editor. The Alkaloids: Chemistry and Pharmacology. Vol. 34. Academic Press; New York: 1989. pp. 1–75.
-
-
-
For reviews on epipolythiodiketopiperazines, see: Waring P, Eichner RD, Müllbacher A. Med. Res. Rev. 1988;8:499.; Waring P, Beaver J. Gen. Pharmac. 1996;27:1311.; Gardiner DM, Waring P, Howlett BJ. Microbiology. 2005;151:1021.; Patron NJ, Waller RF, Cozijnsen AJ, Straney DC, Gardiner DM, Nierman WC, Howlett BJ. BMC Evol. Biol. 2007;7:174.; Huang R, Zhou X, Xu T, Yang X, Liu Y. Chem. Biodiv. 2010;7:2809.; Iwasa E, Hamashima Y, Sodeoka M. Isr. J. Chem. 2011;51:420.
-
-
-
For reviews about pharmacologically active sulfur-containing compounds, see: Řezanka T, Sobotka M, Spížek J, Sigler K. Anti-Infect. Agents Med. Chem. 2006;5:187.; Jiang C-S, Müller WEG, Schröder HC, Guo Y-W. Chem. Rev. 2012 DOI: 10.1021/cr200173z.
-
-
-
For the mechanism of toxicity, see: Chai CLL, Waring P. Redox Rep. 2000;5:257.; Bernardo PH, Chai CLL, Deeble GJ, Liu X-M, Waring P. Bioorg. Med. Chem. Lett. 2001;11:483.; Block KM, Wang H, Szabó LZ, Polaske NW, Henchey LK, Dubey R, Kushal S, László CF, Makhoul J, Song Z, Meuillet EJ, Olenyuk BZ. J. Am. Chem. Soc. 2009;131:18078.; Cook KM, Hilton ST, Mecinović J, Motherwell WB, Figg WD, Schofield CJ. J. Biol. Chem. 2009;284:26831.
-
-
-
For approaches to epidithiodiketopiperazines, see: Trown PW. Biochem. Biophys. Res. Commun. 1968;33:402.; Hino T, Sato T. Tetrahedron Lett. 1971;12:3127.; Poisel H, Schmidt U. Chem. Ber. 1971;104:1714.; Poisel H, Schmidt U. Chem. Ber. 1972;105:625.; Öhler E, Tataruch F, Schmidt U. Chem. Ber. 1973;106:396.; Öhler E, Schmidt U. Chem. Ber. 1975;108:2907.; Ottenheijm HCJ, Herscheid JDM, Kerkhoff GPC, Spande TF. J. Org. Chem. 1976;41:3433.; Coffen DL, Katonak DA, Nelson NR, Sancilio FD. J. Org. Chem. 1977;42:948.; Herscheid JDM, Nivard RJF, Tijhus MW, Ottenheijm HCJ. J. Org. Chem. 1980;45:1885.; Kirby GW, Robins DJ, Stark WM. J. Chem. Soc., Chem. Commun. 1983:812.; Williams RM, Armstrong RW, Maruyama LK, Dung J-S, Anderson OP. J. Am. Chem. Soc. 1985;107:3246.; Aliev AE, Hilton ST, Motherwell WB, Selwood DL. Tetrahedron Lett. 2006;47:2387.; Polaske NW, Dubey R, Nichol GS, Olenyuk B. Tetrahedron: Asym. 2009;20:2742.; Overman LE, Sato T. Org. Lett. 2007;9:5267.; Ruff BM, Zhong S, Nieger M, Bräse S. Org. Biomol. Chem. 2012;10:935.; Nicolaou KC, Giguère D, Totokotsopoulos S, Sun Y-P. Angew. Chem., Int. Ed. 2012;51:728.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

