Length and PKA Dependence of Force Generation and Loaded Shortening in Porcine Cardiac Myocytes
- PMID: 22844597
- PMCID: PMC3398585
- DOI: 10.1155/2012/371415
Length and PKA Dependence of Force Generation and Loaded Shortening in Porcine Cardiac Myocytes
Abstract
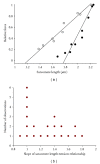
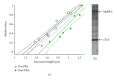
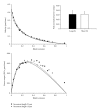
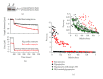
In healthy hearts, ventricular ejection is determined by three myofibrillar properties; force, force development rate, and rate of loaded shortening (i.e., power). The sarcomere length and PKA dependence of these mechanical properties were measured in porcine cardiac myocytes. Permeabilized myocytes were prepared from left ventricular free walls and myocyte preparations were calcium activated to yield ~50% maximal force after which isometric force was measured at varied sarcomere lengths. Porcine myocyte preparations exhibited two populations of length-tension relationships, one being shallower than the other. Moreover, myocytes with shallow length-tension relationships displayed steeper relationships following PKA. Sarcomere length-K(tr) relationships also were measured and K(tr) remained nearly constant over ~2.30 μm to ~1.90 μm and then increased at lengths below 1.90 μm. Loaded-shortening and peak-normalized power output was similar at ~2.30 μm and ~1.90 μm even during activations with the same [Ca(2+)], implicating a myofibrillar mechanism that sustains myocyte power at lower preloads. PKA increased myocyte power and yielded greater shortening-induced cooperative deactivation in myocytes, which likely provides a myofibrillar mechanism to assist ventricular relaxation. Overall, the bimodal distribution of myocyte length-tension relationships and the PKA-mediated changes in myocyte length-tension and power are likely important modulators of Frank-Starling relationships in mammalian hearts.
Figures



Similar articles
-
Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres.J Physiol. 2010 Aug 1;588(Pt 15):2891-903. doi: 10.1113/jphysiol.2010.190504. Epub 2010 Jun 7. J Physiol. 2010. PMID: 20530113 Free PMC article.
-
Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes.Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1524-31. doi: 10.1152/ajpheart.00864.2008. Epub 2009 Feb 27. Am J Physiol Heart Circ Physiol. 2009. PMID: 19252095 Free PMC article.
-
Regulation of Myofilament Contractile Function in Human Donor and Failing Hearts.Front Physiol. 2020 May 25;11:468. doi: 10.3389/fphys.2020.00468. eCollection 2020. Front Physiol. 2020. PMID: 32523542 Free PMC article.
-
The interdependence of Ca2+ activation, sarcomere length, and power output in the heart.Pflugers Arch. 2011 Jul;462(1):61-7. doi: 10.1007/s00424-011-0949-y. Epub 2011 Mar 15. Pflugers Arch. 2011. PMID: 21404040 Free PMC article. Review.
-
Cardiac function and modulation of sarcomeric function by length.Cardiovasc Res. 2008 Mar 1;77(4):627-36. doi: 10.1093/cvr/cvm099. Epub 2007 Dec 12. Cardiovasc Res. 2008. PMID: 18079105 Review.
Cited by
-
The contribution of cardiac myosin binding protein-c Ser282 phosphorylation to the rate of force generation and in vivo cardiac contractility.J Physiol. 2014 Sep 1;592(17):3747-65. doi: 10.1113/jphysiol.2014.276022. Epub 2014 Jun 20. J Physiol. 2014. PMID: 24951619 Free PMC article.
-
Molecule specific effects of PKA-mediated phosphorylation on rat isolated heart and cardiac myofibrillar function.Arch Biochem Biophys. 2016 Jul 1;601:22-31. doi: 10.1016/j.abb.2016.01.019. Epub 2016 Feb 15. Arch Biochem Biophys. 2016. PMID: 26854722 Free PMC article.
-
Thin filament regulation of cardiac muscle power output: Implications for targets to improve human failing hearts.J Gen Physiol. 2023 May 1;155(5):e202213290. doi: 10.1085/jgp.202213290. Epub 2023 Mar 31. J Gen Physiol. 2023. PMID: 37000170 Free PMC article.
-
Titin-mediated control of cardiac myofibrillar function.Arch Biochem Biophys. 2014 Jun 15;552-553:83-91. doi: 10.1016/j.abb.2013.11.005. Epub 2013 Nov 20. Arch Biochem Biophys. 2014. PMID: 24269766 Free PMC article.
-
Hypothesis and theory: mechanical instabilities and non-uniformities in hereditary sarcomere myopathies.Front Physiol. 2014 Sep 15;5:350. doi: 10.3389/fphys.2014.00350. eCollection 2014. Front Physiol. 2014. PMID: 25309450 Free PMC article.
References
-
- Fabiato A, Fabiato F. Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature . 1975;256(5512):54–56. - PubMed
-
- Rossmanith GH, Hoh JFY, Kirman A, Kwan LJ. Influence of V1 and V3 isomyosins on the mechanical behaviour of rat papillary muscle as studied by pseudo-random binary noise modulated length perturbations. Journal of Muscle Research and Cell Motility . 1986;7(4):307–319. - PubMed
-
- De Tombe PP, Ter Keurs HEDJ. Force and velocity of sarcomere shortening in trabeculae from rat heart. Effects of temperature. Circulation Research . 1990;66(5):1239–1254. - PubMed
-
- Puceat M, Clement O, Lechene P, Pelosin JM, Ventura-Clapier R, Vassort G. Neurohormonal control of calcium sensitivity of myofilaments in rat single heart cells. Circulation Research . 1990;67(2):517–524. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

