Cost-effectiveness comparison of response strategies to a large-scale anthrax attack on the chicago metropolitan area: impact of timing and surge capacity
- PMID: 22845046
- PMCID: PMC3440066
- DOI: 10.1089/bsp.2011.0105
Cost-effectiveness comparison of response strategies to a large-scale anthrax attack on the chicago metropolitan area: impact of timing and surge capacity
Abstract
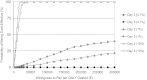
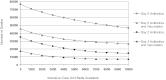
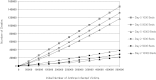
Rapid public health response to a large-scale anthrax attack would reduce overall morbidity and mortality. However, there is uncertainty about the optimal cost-effective response strategy based on timing of intervention, public health resources, and critical care facilities. We conducted a decision analytic study to compare response strategies to a theoretical large-scale anthrax attack on the Chicago metropolitan area beginning either Day 2 or Day 5 after the attack. These strategies correspond to the policy options set forth by the Anthrax Modeling Working Group for population-wide responses to a large-scale anthrax attack: (1) postattack antibiotic prophylaxis, (2) postattack antibiotic prophylaxis and vaccination, (3) preattack vaccination with postattack antibiotic prophylaxis, and (4) preattack vaccination with postattack antibiotic prophylaxis and vaccination. Outcomes were measured in costs, lives saved, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). We estimated that postattack antibiotic prophylaxis of all 1,390,000 anthrax-exposed people beginning on Day 2 after attack would result in 205,835 infected victims, 35,049 fulminant victims, and 28,612 deaths. Only 6,437 (18.5%) of the fulminant victims could be saved with the existing critical care facilities in the Chicago metropolitan area. Mortality would increase to 69,136 if the response strategy began on Day 5. Including postattack vaccination with antibiotic prophylaxis of all exposed people reduces mortality and is cost-effective for both Day 2 (ICER=$182/QALY) and Day 5 (ICER=$1,088/QALY) response strategies. Increasing ICU bed availability significantly reduces mortality for all response strategies. We conclude that postattack antibiotic prophylaxis and vaccination of all exposed people is the optimal cost-effective response strategy for a large-scale anthrax attack. Our findings support the US government's plan to provide antibiotic prophylaxis and vaccination for all exposed people within 48 hours of the recognition of a large-scale anthrax attack. Future policies should consider expanding critical care capacity to allow for the rescue of more victims.
Figures
Similar articles
-
Responding to a small-scale bioterrorist anthrax attack: cost-effectiveness analysis comparing preattack vaccination with postattack antibiotic treatment and vaccination.Arch Intern Med. 2007 Apr 9;167(7):655-62. doi: 10.1001/archinte.167.7.655. Arch Intern Med. 2007. PMID: 17420423
-
Cost-effectiveness of defending against bioterrorism: a comparison of vaccination and antibiotic prophylaxis against anthrax.Ann Intern Med. 2005 Apr 19;142(8):601-10. doi: 10.7326/0003-4819-142-8-200504190-00008. Ann Intern Med. 2005. PMID: 15838066
-
Summaries for patients. What is the most cost-effective way to protect people in the event of an anthrax terror attack?Ann Intern Med. 2005 Apr 19;142(8):I40. doi: 10.7326/0003-4819-142-8-200504190-00002. Ann Intern Med. 2005. PMID: 15838060 No abstract available.
-
Vaccines and bioterrorism: smallpox and anthrax.J Fam Pract. 2003 Jan;52(1 Suppl):S56-61. J Fam Pract. 2003. PMID: 12556279 Review.
-
The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justifiable?Emerg Infect Dis. 1997 Apr-Jun;3(2):83-94. doi: 10.3201/eid0302.970201. Emerg Infect Dis. 1997. PMID: 9204289 Free PMC article. Review.
Cited by
-
Estimation of Time Period for Effective Human Inhalational Anthrax Treatment Including Antitoxin Therapy.PLoS Curr. 2017 Jul 28;9:ecurrents.outbreaks.7896c43f69838f17ce1c2c372e79d55d. doi: 10.1371/currents.outbreaks.7896c43f69838f17ce1c2c372e79d55d. PLoS Curr. 2017. PMID: 28856066 Free PMC article.
-
United States' regulatory approved pharmacotherapies for nuclear reactor explosions and anthrax-associated bioterrorism.Expert Opin Drug Saf. 2023 Jul-Dec;22(9):783-788. doi: 10.1080/14740338.2023.2245748. Epub 2023 Aug 18. Expert Opin Drug Saf. 2023. PMID: 37594915 Free PMC article. Review.
References
-
- Inglesby TV. O'Toole T. Henderson DA, et al. Anthrax as a biological weapon, 2002. JAMA. 2002;287:2236–2252. - PubMed
-
- Brachman PS. Bioterrorism: an update with a focus on anthrax. Am J Epidemiol. 2002;155:981–987. - PubMed
-
- Inglesby TV. O'Toole T. Henderson DA. Preventing the use of biological weapons: improving response should prevention fail. Clin Infect Dis. 2000;30:926–929. - PubMed
-
- World Health Organization. Health Aspects of Chemical and Biological Weapons: Report of a WHO Group of Consultants. Geneva, Switzerland: World Health Organization; 1970.
-
- Office of Technology Assessment, US Congress. Proliferation of Weapons of Mass Destruction: Assessing the Risks. Washington, DC: Office of Technology Assessment; 1993. [Jun 7;2012 ].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

