Regulation of apoptosis in HL-1 cardiomyocytes by phosphorylation of the receptor tyrosine kinase EphA2 and protection by lithocholic acid
- PMID: 22845314
- PMCID: PMC3514767
- DOI: 10.1111/j.1476-5381.2012.02117.x
Regulation of apoptosis in HL-1 cardiomyocytes by phosphorylation of the receptor tyrosine kinase EphA2 and protection by lithocholic acid
Abstract
Background and purpose: Heart failure and atrial fibrillation are associated with apoptosis of cardiomyocytes, suggesting common abnormalities in pro-apoptotic cardiac molecules. Activation of the receptor tyrosine kinase EphA2 causes apoptosis in vitro, and dysregulation of EphA2-dependent signalling is implicated in LEOPARD and Noonan syndromes associated with cardiomyopathy. Molecular pathways and regulation of EphA2 signalling in the heart are poorly understood. Here we elucidated the pathways of EphA2-dependent apoptosis and evaluated a therapeutic strategy to prevent EphA2 activation and cardiac cell death.
Experimental approach: EphA2 signalling was studied in an established model of doxazosin-induced apoptosis in HL-1 cells. Apoptosis was measured with TUNEL assays and as cell viability using a formazan method. Western blotting and siRNA for EphA2 were also used.
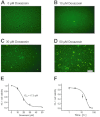
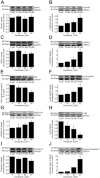
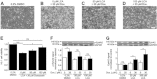
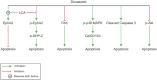
Key results: Apoptosis induced by doxazosin (EC(50) = 17.3 μM) was associated with EphA2 activation through enhanced phosphorylation (2.2-fold). Activation of pro-apoptotic downstream factors, phospho-SHP-2 (3.9-fold), phospho-p38 MAPK (2.3-fold) and GADD153 (1.6-fold) resulted in cleavage of caspase 3. Furthermore, two anti-apoptotic enzymes were suppressed (focal adhesion kinase, by 41%; phospho-Akt, by 78%). Inactivation of EphA2 with appropriate siRNA mimicked pro-apoptotic effects of doxazosin. Finally, administration of lithocholic acid (LCA) protected against apoptosis by increasing EphA2 protein levels and decreasing EphA2 phosphorylation.
Conclusions and implications: EphA2 phosphorylation and activation of SHP-2 are critical steps in apoptosis. Reduction of EphA2 phosphorylation by LCA may represent a novel approach for future anti-apoptotic treatment of heart failure and atrial fibrillation.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

References
-
- Aimé-Sempé C, Folliguet T, Rücker-Martin C, Krajewska M, Krajewska S, Heimburger M, et al. Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol. 1999;34:1577–1586. - PubMed
-
- Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, et al. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218–225. - PubMed
-
- Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

