Glucose-dependent trafficking of 5-HT3 receptors in rat gastrointestinal vagal afferent neurons
- PMID: 22845622
- PMCID: PMC3440531
- DOI: 10.1111/j.1365-2982.2012.01987.x
Glucose-dependent trafficking of 5-HT3 receptors in rat gastrointestinal vagal afferent neurons
Abstract
Background: Intestinal glucose induces gastric relaxation via vagally mediated sensory-motor reflexes. Glucose can alter the activity of gastrointestinal (GI) vagal afferent (sensory) neurons directly, via closure of ATP-sensitive potassium channels, and indirectly, via the release of 5-hydroxytryptamine (5-HT) from mucosal enteroendocrine cells. We hypothesized that glucose may also be able to modulate the ability of GI vagal afferent neurons to respond to the released 5-HT, via regulation of neuronal 5-HT(3) receptors.
Methods: Whole-cell patch clamp recordings were made from acutely dissociated GI-projecting vagal afferent neurons exposed to equiosmolar Krebs' solution containing different concentrations of d-glucose (1.25-20 mmol L(-1)) and the response to picospritz application of 5-HT assessed. The distribution of 5-HT(3) receptors in neurons exposed to different glucose concentrations was also assessed immunohistochemically.
Key results: Increasing or decreasing extracellular d-glucose concentration increased or decreased, respectively, the 5-HT-induced inward current and the proportion of 5-HT(3) receptors associated with the neuronal membrane. These responses were blocked by the Golgi-disrupting agent Brefeldin-A (5 μmol L(-1)) suggesting involvement of a protein-trafficking pathway. Furthermore, l-glucose did not mimic the response of d-glucose implying that metabolic events downstream of neuronal glucose uptake are required to observe the modulation of 5-HT(3) receptor mediated responses.
Conclusions & inferences: These results suggest that, in addition to inducing the release of 5-HT from enterochromaffin cells, glucose may also increase the ability of GI vagal sensory neurons to respond to the released 5-HT, providing a means by which the vagal afferent signal can be amplified or prolonged.
© 2012 Blackwell Publishing Ltd.
Figures
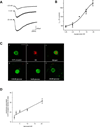
Electrophysiological traces illustrating the 5-HT-induced inward current in a single GI-projecting vagal afferent neuron voltage clamped at -50mV. Superfusion with equiosmolar Krebs’ solution containing different concentrations of glucose modulated the amplitude of the 5-HT-induced inward current; decreasing the glucose concentration to 1.25mM decreased the magnitude of the inward current whereas increasing the glucose concentration to 20mM increased the magnitude of the inward current.
Concentration-response curve illustrating the modulation of the 5-HT-induced inward current in GI vagal afferent neurons by glucose. Neurons were voltage clamped at −50mV prior to picrospritz application of 5-HT; each neuron was exposed to equiosmolar Krebs’ solution containing at least 3 different concentrations of glucose. The 5-HT-induced inward current was normalized to the amplitude of the current induced in the presence of 5mM glucose. N=6–41 for each point.
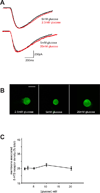
Photomicrographs of representative confocal images (1µm thick) of GI-projecting vagal afferent neurons (5-HT3 receptors - green; DiI - red). (a) an acutely dissociated vagal afferent neuron illustrating 5-HT3 receptor distribution (20mM glucose); (b) the same neuron as in (a) depicting DiI-labeling from the GI-tract; (c) merged image. 5-HT3 receptor distribution in acutely dissociated GI-projecting vagal afferent neurons following exposure to 1.25mM (d), 5mM (e) or 20mM (f) glucose prior to fixation. Note that as the extracellular glucose concentration was elevated, membrane-associate 5-HT3 receptor-density increased. Calibration bar = 20µm.
Concentration-response curve illustrating the modulation of 5-HT3 receptor density in GI vagal afferent neurons induced by extracellular glucose concentration. 5-HT3 receptor density associated with the neuronal membrane was expressed as a proportion of that neurons’ total 5-HT3 receptor density. Note that an increase in glucose level was associated with a linear increase in 5-HT3 receptor density associated with the neuronal membrane (r2 = 0.97).
Electrophysiological traces illustrating the 5-HT-induced inward current in a single a vagal afferent neuron labeled following application of DiI to the SLN. The neuron was voltage clamped at -50mV prior to picospritz application of 5-HT. Superfusion with equiosmolar Krebs’ solution containing either 1.25 or 20mM glucose failed to modulate the amplitude of the 5-HT-induced inward current.
Photomicrographs of representative confocal images (1µm thick) of acutely-dissociated SLN-labled vagal afferent neurons illustrating 5-HT3 receptor distribution (green). Neurons were exposed to 1.25, 5 or 20mM glucose prior to fixation. Note that there was no alteration in 5-HT3 receptor distribution in response to change in extracellular glucose concentration. Calibration bar = 20µm.
Concentration-response curve illustrating the modulation of 5-HT3 receptor density in SLN-labeled vagal afferent neurons in response to extracellular glucose concentration. 5-HT3 receptor density associated with the neuronal membrane was expressed as a proportion of that neurons’ total 5-HT3 receptor density. Note that extracellular glucose concentration had no effect upon the proportion of membrane-associated 5-HT3 receptor density.
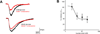
Electrophysiological traces illustrating the effects of the 5-HT3 receptor selective antagonist, ondansetron (0.1µM) to inhibit the 5-HT induced inward current in a GI-projecting vagal afferent neuron. The neuron was voltage clamped at -50mV prior to picospritz application of 5-HT. Ondansetron decreased the amplitude of the 5-HT-induced inward current. Superfusion of that same neuron with equiosmolar Krebs’ solution containing 20mM glucose increased the amplitude of the 5-HT-induced current; at this higher concentration of glucose, ondansetron was less effective at inhibiting the 5-HT-induced inward current.
Concentration-response curve illustrating the actions of extracellular glucose concentration to modulate the ability of ondansetron (0.1µM) to inhibit the 5-HT-induced inward current in GI vagal afferent neurons. Note that as the extracellular glucose concentration increased, ondansetron became less effective at inhibiting the 5-HT-induced inward current, suggesting that as extracellular glucose levels increased, the number of membrane-associated 5-HT3 receptors also increased.
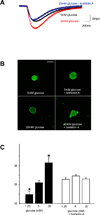
Electrophysiological traces illustrating the effects of the Golgi-disrupting agent, brefeldin-A (5µM) to block the glucose-induced alteration in 5-HT-mediated inward current in a GI-projecting vagal afferent neuron. The neuron was voltage clamped at -50mM prior to picospritz application of 5-HT (black trace). The magnitude of the 5-HT-induced inward current was increased following superfusion with equiosmolar Krebs’ solution containing 20mM glucose (red trace). Following wash-out and recovery, however, superfusion with brefeldin-A inhibited the ability of 20mM glucose to increase the response to 5-HT (blue trace), suggesting involvement of a protein trafficking pathway.
Photomicrographs of representative confocal images (1µm thick) of acutely-dissociated GI-projecting vagal afferent neurons illustrating 5-HT3 receptor distribution (green). Neurons were exposed to either 5 or 20mM glucose in the absence or presence of the Golgi-disrupting agent brefeldin-A (5µM) prior to fixation. Note that exposure to brefeldin-A inhibited the ability of 20mM glucose to increase the proportion of 5-HT3 receptors associated with the neuronal membrane, suggesting involvement of a protein-trafficking pathway. Calibration bar = 20µm.
Graphical representation of the effects of brefeldin-A (5µM) to disrupt to ability of extracellular glucose to modulate the proportion of 5-HT3 receptors associated with the membrane of GI vagal afferent neurons. In control conditions, varying the extracellular glucose concentration to 1.25 or 20mM, respectively, altered the proportion of 5-HT3 receptors associated with the neuronal membrane (black bars). In the presence of brefeldin-A, however, altering extracellular glucose concentration had no effect upon membrane-associated 5-HT3 receptor density. *P<0.05 vs control (5mM) glucose.

Electrophysiological traces illustrating the effects of D- and L-glucose on the response to 5-HT in a GI-projectin vagal afferent neuron. The neuron was voltage-clamped at −50mV prior to picospritz application of 5-HT (black trace). Superfusion with equiosmolar Krebs’ solution containing 20mM D-glucose increased the magnitude of the 5-HT-induced inward current (red trace) whereas superfusion with 5mM D-glucose + 15mM L-glucose had no effect (blue trace).
Graphical representation of the effects of D- and L-glucose on the proportion of 5-HT3 receptors associated with the membrane of GI-projecting vagal afferent neurons. Neurons were exposed to 5 or 20mM D-glucose or 5mM D-glucose + 15mM L-glucose prior to fixation. Elevating the extracellular D-glucose concentration increased the proportion of 5-HT3 receptors associated with the neuronal membrane whereas elevating the extracellular L-glucose concentration had no effect. These results suggest that the effects of glucose are dependent upon glucose metabolism, rather than uptake, within the vagal afferent neurons. *p,0.05 vs control (5mM) glucose.
References
-
- MacGregor IL, Gueller R, Watts HD, Meyer JH. The effect of acute hyperglycemia on gastric emptying in man. Gastroenterology. 1976 Feb;70(2):190–196. - PubMed
-
- Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001 Feb;24(2):371–381. - PubMed
-
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259(28):H1307–H1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

