Pharmacokinetic and -dynamic modelling of G-CSF derivatives in humans
- PMID: 22846180
- PMCID: PMC3507764
- DOI: 10.1186/1742-4682-9-32
Pharmacokinetic and -dynamic modelling of G-CSF derivatives in humans
Abstract
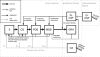
Background: The human granulocyte colony-stimulating factor (G-CSF) is routinely applied to support recovery of granulopoiesis during the course of cytotoxic chemotherapies. However, optimal use of the drug is largely unknown. We showed in the past that a biomathematical compartment model of human granulopoiesis can be used to make clinically relevant predictions regarding new, yet untested chemotherapy regimen. In the present paper, we aim to extend this model by a detailed pharmacokinetic and -dynamic modelling of two commonly used G-CSF derivatives Filgrastim and Pegfilgrastim.
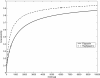
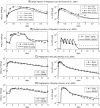
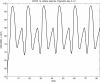
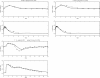
Results: Model equations are based on our physiological understanding of the drugs which are delayed absorption of G-CSF when applied to the subcutaneous tissue, dose-dependent bioavailability, unspecific first order elimination, specific elimination in dependence on granulocyte counts and reversible protein binding. Pharmacokinetic differences between Filgrastim and Pegfilgrastim were modelled as different parameter sets. Our former cell-kinetic model of granulopoiesis was essentially preserved, except for a few additional assumptions and simplifications. We assumed a delayed action of G-CSF on the bone marrow, a delayed action of chemotherapy and differences between Filgrastim and Pegfilgrastim with respect to stimulation potency of the bone marrow. Additionally, we incorporated a model of combined action of Pegfilgrastim and Filgrastim or endogenous G-CSF which interact via concurrent receptor binding. Unknown pharmacokinetic or cell-kinetic parameters were determined by fitting the predictions of the model to available datasets of G-CSF applications, chemotherapy applications or combinations of it. Data were either extracted from the literature or were received from cooperating clinical study groups. Model predictions fitted well to both, datasets used for parameter estimation and validation scenarios as well. A unique set of parameters was identified which is valid for all scenarios considered. Differences in pharmacokinetic parameter estimates between Filgrastim and Pegfilgrastim were biologically plausible throughout.
Conclusion: We conclude that we established a comprehensive biomathematical model to explain the dynamics of granulopoiesis under chemotherapy and applications of two different G-CSF derivatives. We aim to apply the model to a large variety of chemotherapy regimen in the future in order to optimize corresponding G-CSF schedules or to individualize G-CSF treatment according to the granulotoxic risk of a patient.
Figures
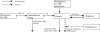
Similar articles
-
A combined model of human erythropoiesis and granulopoiesis under growth factor and chemotherapy treatment.Theor Biol Med Model. 2014 May 26;11:24. doi: 10.1186/1742-4682-11-24. Theor Biol Med Model. 2014. PMID: 24886056 Free PMC article.
-
A pharmacokinetic model of filgrastim and pegfilgrastim application in normal mice and those with cyclophosphamide-induced granulocytopaenia.Cell Prolif. 2009 Dec;42(6):813-22. doi: 10.1111/j.1365-2184.2009.00638.x. Epub 2009 Aug 17. Cell Prolif. 2009. PMID: 19689472 Free PMC article.
-
Model-based optimization of G-CSF treatment during cytotoxic chemotherapy.J Cancer Res Clin Oncol. 2018 Feb;144(2):343-358. doi: 10.1007/s00432-017-2540-1. Epub 2017 Nov 4. J Cancer Res Clin Oncol. 2018. PMID: 29103159 Free PMC article.
-
Pharmacokinetics and pharmacodynamics of pegfilgrastim.Clin Pharmacokinet. 2011 May;50(5):295-306. doi: 10.2165/11586040-000000000-00000. Clin Pharmacokinet. 2011. PMID: 21456630 Review.
-
Pharmacokinetics of pegfilgrastim.Pharmacotherapy. 2003 Aug;23(8 Pt 2):9S-14S. doi: 10.1592/phco.23.9.9s.32888. Pharmacotherapy. 2003. PMID: 12921217 Review.
Cited by
-
Ycasd - a tool for capturing and scaling data from graphical representations.BMC Bioinformatics. 2014 Jun 25;15:219. doi: 10.1186/1471-2105-15-219. BMC Bioinformatics. 2014. PMID: 24965054 Free PMC article.
-
A combined model of human erythropoiesis and granulopoiesis under growth factor and chemotherapy treatment.Theor Biol Med Model. 2014 May 26;11:24. doi: 10.1186/1742-4682-11-24. Theor Biol Med Model. 2014. PMID: 24886056 Free PMC article.
-
Expression and function of hematopoiesis-stimulating factor receptors on the GPI- and GPI+ hematopoietic stem cells of patients with paroxysmal nocturnal hemoglobinuria/aplastic anemia syndrome.Exp Ther Med. 2016 May;11(5):1668-1672. doi: 10.3892/etm.2016.3163. Epub 2016 Mar 15. Exp Ther Med. 2016. PMID: 27168787 Free PMC article.
-
Modelling chemotherapy effects on granulopoiesis.BMC Syst Biol. 2014 Dec 24;8:138. doi: 10.1186/s12918-014-0138-7. BMC Syst Biol. 2014. PMID: 25539928 Free PMC article.
-
Steady-state volume of distribution of two-compartment models with simultaneous linear and saturated elimination.J Pharmacokinet Pharmacodyn. 2016 Aug;43(4):447-59. doi: 10.1007/s10928-016-9483-z. Epub 2016 Jul 12. J Pharmacokinet Pharmacodyn. 2016. PMID: 27405818
References
-
- Crawford J. Pegfilgrastim administered once per cycle reduces incidence of chemotherapy-induced neutropenia. Drugs. 2002;62(Suppl 1):89–98. - PubMed
-
- Dale D. Current management of chemotherapy-induced neutropenia: the role of colony-stimulating factors. Semin Oncol. 2003;30:3–9. - PubMed
-
- Siena S, Secondino S, Giannetta L, Carminati O, Pedrazzoli P. Optimising management of neutropenia and anaemia in cancer chemotherapy-advances in cytokine therapy. Crit Rev Oncol Hematol. 2003;48:S39–s47. - PubMed
-
- Wunderlich A, Kloess M, Reiser M, Rudolph C, Truemper L, Bittner S, Schmalenberg H, Schmits R, Pfreundschuh M, Loeffler M. German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL): Practicability and acute haematological toxicity of 2- and 3-weekly CHOP and CHOEP chemotherapy for aggressive non-Hodgkin’s lymphoma: results from the NHL-B trial of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) Ann Oncol. 2003;14:881–893. - PubMed
-
- Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, Chazin VR, Bruszewski J, Lu H, Chen KK, Barendt J, Platzer E, Moore MAS, Mertelsmann R, Welte K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232:61–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

