Antisense reduction of NADP-malic enzyme in Flaveria bidentis reduces flow of CO2 through the C4 cycle
- PMID: 22846191
- PMCID: PMC3461530
- DOI: 10.1104/pp.112.203240
Antisense reduction of NADP-malic enzyme in Flaveria bidentis reduces flow of CO2 through the C4 cycle
Abstract
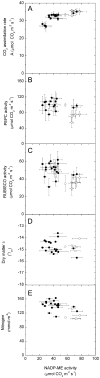
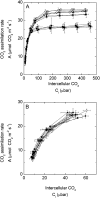
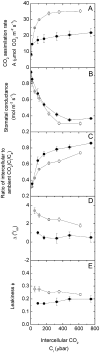
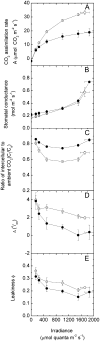
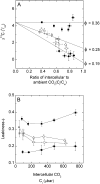
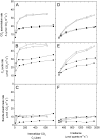
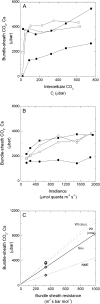
An antisense construct targeting the C(4) isoform of NADP-malic enzyme (ME), the primary enzyme decarboxylating malate in bundle sheath cells to supply CO(2) to Rubisco, was used to transform the dicot Flaveria bidentis. Transgenic plants (α-NADP-ME) exhibited a 34% to 75% reduction in NADP-ME activity relative to the wild type with no visible growth phenotype. We characterized the effect of reducing NADP-ME on photosynthesis by measuring in vitro photosynthetic enzyme activity, gas exchange, and real-time carbon isotope discrimination (Δ). In α-NADP-ME plants with less than 40% of wild-type NADP-ME activity, CO(2) assimilation rates at high intercellular CO(2) were significantly reduced, whereas the in vitro activities of both phosphoenolpyruvate carboxylase and Rubisco were increased. Δ measured concurrently with gas exchange in these plants showed a lower Δ and thus a lower calculated leakiness of CO(2) (the ratio of CO(2) leak rate from the bundle sheath to the rate of CO(2) supply). Comparative measurements on antisense Rubisco small subunit F. bidentis plants showed the opposite effect of increased Δ and leakiness. We use these measurements to estimate the C(4) cycle rate, bundle sheath leak rate, and bundle sheath CO(2) concentration. The comparison of α-NADP-ME and antisense Rubisco small subunit demonstrates that the coordination of the C(3) and C(4) cycles that exist during environmental perturbations by light and CO(2) can be disrupted through transgenic manipulations. Furthermore, our results suggest that the efficiency of the C(4) pathway could potentially be improved through a reduction in C(4) cycle activity or increased C(3) cycle activity.
Figures
Similar articles
-
Photosynthesis of C3, C3-C4, and C4 grasses at glacial CO2.J Exp Bot. 2014 Jul;65(13):3669-81. doi: 10.1093/jxb/eru155. Epub 2014 Apr 10. J Exp Bot. 2014. PMID: 24723409 Free PMC article.
-
Photosynthetic flexibility in maize exposed to salinity and shade.J Exp Bot. 2014 Jul;65(13):3715-24. doi: 10.1093/jxb/eru130. Epub 2014 Apr 1. J Exp Bot. 2014. PMID: 24692650 Free PMC article.
-
Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry.J Exp Bot. 2010 Sep;61(14):4109-22. doi: 10.1093/jxb/erq226. Epub 2010 Aug 6. J Exp Bot. 2010. PMID: 20693408 Free PMC article.
-
The efficiency of C(4) photosynthesis under low light conditions: assumptions and calculations with CO(2) isotope discrimination.J Exp Bot. 2011 May;62(9):3119-34. doi: 10.1093/jxb/err073. Epub 2011 Apr 28. J Exp Bot. 2011. PMID: 21527629 Review.
-
Overexpression of C(4)-cycle enzymes in transgenic C(3) plants: a biotechnological approach to improve C(3)-photosynthesis.J Exp Bot. 2002 Apr;53(369):591-607. doi: 10.1093/jexbot/53.369.591. J Exp Bot. 2002. PMID: 11886879 Review.
Cited by
-
Getting the most out of natural variation in C4 photosynthesis.Photosynth Res. 2014 Feb;119(1-2):157-67. doi: 10.1007/s11120-013-9872-8. Epub 2013 Jun 21. Photosynth Res. 2014. PMID: 23794170 Review.
-
Installation of C4 photosynthetic pathway enzymes in rice using a single construct.Plant Biotechnol J. 2021 Mar;19(3):575-588. doi: 10.1111/pbi.13487. Epub 2020 Oct 27. Plant Biotechnol J. 2021. PMID: 33016576 Free PMC article.
-
Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation.Photosynth Res. 2014 Feb;119(1-2):101-17. doi: 10.1007/s11120-013-9874-6. Epub 2013 Jun 26. Photosynth Res. 2014. PMID: 23801171 Review.
-
Photosynthesis of C3, C3-C4, and C4 grasses at glacial CO2.J Exp Bot. 2014 Jul;65(13):3669-81. doi: 10.1093/jxb/eru155. Epub 2014 Apr 10. J Exp Bot. 2014. PMID: 24723409 Free PMC article.
-
Carbon isotope discrimination as a diagnostic tool for C4 photosynthesis in C3-C4 intermediate species.J Exp Bot. 2016 May;67(10):3109-21. doi: 10.1093/jxb/erv555. Epub 2016 Feb 8. J Exp Bot. 2016. PMID: 26862154 Free PMC article.
References
-
- Ashton AR. (1997) NADP-malic enzyme from the C4 plant Flaveria bidentis: nucleotide substrate specificity. Arch Biochem Biophys 345: 251–258 - PubMed
-
- Berry JA, Farquhar GD. (1978) The CO2 concentrating function of C4 photosynthesis: a biochemical model. In D Hall, J Coombs, T Goodwin, eds, The Proceedings of the Fourth International Congress on Photosynthesis. Biochemical Society of London, London, pp 119–131
-
- Chitty JA, Furbank RT, Marshall JS, Chen Z, Taylor WC. (1994) Genetic transformation of the C4 plant, Flaveria bidentis. Plant J 6: 949–956
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

