Transparent testa16 plays multiple roles in plant development and is involved in lipid synthesis and embryo development in canola
- PMID: 22846192
- PMCID: PMC3461570
- DOI: 10.1104/pp.112.198713
Transparent testa16 plays multiple roles in plant development and is involved in lipid synthesis and embryo development in canola
Erratum in
- Plant Physiol. 2013 Mar;161(3):1584
Abstract
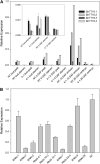
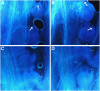
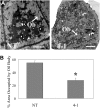
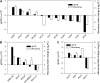
Transparent Testa16 (TT16), a transcript regulator belonging to the B(sister) MADS box proteins, regulates proper endothelial differentiation and proanthocyanidin accumulation in the seed coat. Our understanding of its other physiological roles, however, is limited. In this study, the physiological and developmental roles of TT16 in an important oil crop, canola (Brassica napus), were dissected by a loss-of-function approach. RNA interference (RNAi)-mediated down-regulation of tt16 in canola caused dwarf phenotypes with a decrease in the number of inflorescences, flowers, siliques, and seeds. Fluorescence microscopy revealed that tt16 deficiency affects pollen tube guidance, resulting in reduced fertility and negatively impacting embryo and seed development. Moreover, Bntt16 RNAi plants had reduced oil content and altered fatty acid composition. Transmission electron microscopy showed that the seeds of the RNAi plants had fewer oil bodies than the nontransgenic plants. In addition, tt16 RNAi transgenic lines were more sensitive to auxin. Further analysis by microarray showed that tt16 down-regulation alters the expression of genes involved in gynoecium and embryo development, lipid metabolism, auxin transport, and signal transduction. The broad regulatory function of TT16 at the transcriptional level may explain the altered phenotypes observed in the transgenic lines. Overall, the results uncovered important biological roles of TT16 in plant development, especially in fatty acid synthesis and embryo development.
Figures




Similar articles
-
Brassica napus TT16 homologs with different genomic origins and expression levels encode proteins that regulate a broad range of endothelium-associated genes at the transcriptional level.Plant J. 2013 May;74(4):663-77. doi: 10.1111/tpj.12151. Epub 2013 Mar 14. Plant J. 2013. PMID: 23425240
-
Transcriptional profiling of canola developing embryo and identification of the important roles of BnDof5.6 in embryo development and fatty acids synthesis.Plant Cell Physiol. 2015 Aug;56(8):1624-40. doi: 10.1093/pcp/pcv074. Epub 2015 Jun 19. Plant Cell Physiol. 2015. PMID: 26092973
-
The TRANSPARENT TESTA16 locus encodes the ARABIDOPSIS BSISTER MADS domain protein and is required for proper development and pigmentation of the seed coat.Plant Cell. 2002 Oct;14(10):2463-79. doi: 10.1105/tpc.004127. Plant Cell. 2002. PMID: 12368498 Free PMC article.
-
Enhanced seed oil production in canola by conditional expression of Brassica napus LEAFY COTYLEDON1 and LEC1-LIKE in developing seeds.Plant Physiol. 2011 Jul;156(3):1577-88. doi: 10.1104/pp.111.175000. Epub 2011 May 11. Plant Physiol. 2011. PMID: 21562329 Free PMC article.
-
Genetic enhancement of Brassica napus seed quality.Transgenic Res. 2014 Feb;23(1):39-52. doi: 10.1007/s11248-013-9742-3. Epub 2013 Aug 27. Transgenic Res. 2014. PMID: 23979711 Review.
Cited by
-
Embryonal Control of Yellow Seed Coat Locus ECY1 Is Related to Alanine and Phenylalanine Metabolism in the Seed Embryo of Brassica napus.G3 (Bethesda). 2016 Apr 7;6(4):1073-81. doi: 10.1534/g3.116.027110. G3 (Bethesda). 2016. PMID: 26896439 Free PMC article.
-
Gymnosperm B-sister genes may be involved in ovule/seed development and, in some species, in the growth of fleshy fruit-like structures.Ann Bot. 2013 Aug;112(3):535-44. doi: 10.1093/aob/mct124. Epub 2013 Jun 11. Ann Bot. 2013. PMID: 23761686 Free PMC article.
-
An annotated database of Arabidopsis mutants of acyl lipid metabolism.Plant Cell Rep. 2015 Apr;34(4):519-32. doi: 10.1007/s00299-014-1710-8. Epub 2014 Dec 10. Plant Cell Rep. 2015. PMID: 25487439 Free PMC article.
-
Molecular mechanism of manipulating seed coat coloration in oilseed Brassica species.J Appl Genet. 2013 May;54(2):135-45. doi: 10.1007/s13353-012-0132-y. Epub 2013 Jan 18. J Appl Genet. 2013. PMID: 23329015 Review.
-
Analysis of global gene expression profiles to identify differentially expressed genes critical for embryo development in Brassica rapa.Plant Mol Biol. 2014 Nov;86(4-5):425-42. doi: 10.1007/s11103-014-0238-1. Epub 2014 Sep 12. Plant Mol Biol. 2014. PMID: 25214014
References
-
- Alandete-Saez M, Ron M, McCormick S. (2008) GEX3, expressed in the male gametophyte and in the egg cell of Arabidopsis thaliana, is essential for micropylar pollen tube guidance and plays a role during early embryogenesis. Mol Plant 1: 586–598 - PubMed
-
- Baud S, Lepiniec L. (2009) Regulation of de novo fatty acid synthesis in maturing oilseeds of Arabidopsis. Plant Physiol Biochem 47: 448–455 - PubMed
-
- Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B. (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50: 825–838 - PubMed
-
- Baud S, Wuillème S, To A, Rochat C, Lepiniec L. (2009) Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J 60: 933–947 - PubMed
-
- Becker A, Kaufmann K, Freialdenhoven A, Vincent C, Li M, Saedler H, Theissen G. (2002) A novel MADS-box gene subfamily with a sister-group relationship to class B floral homeotic genes. Mol Genet Genomics 266: 942–950 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

